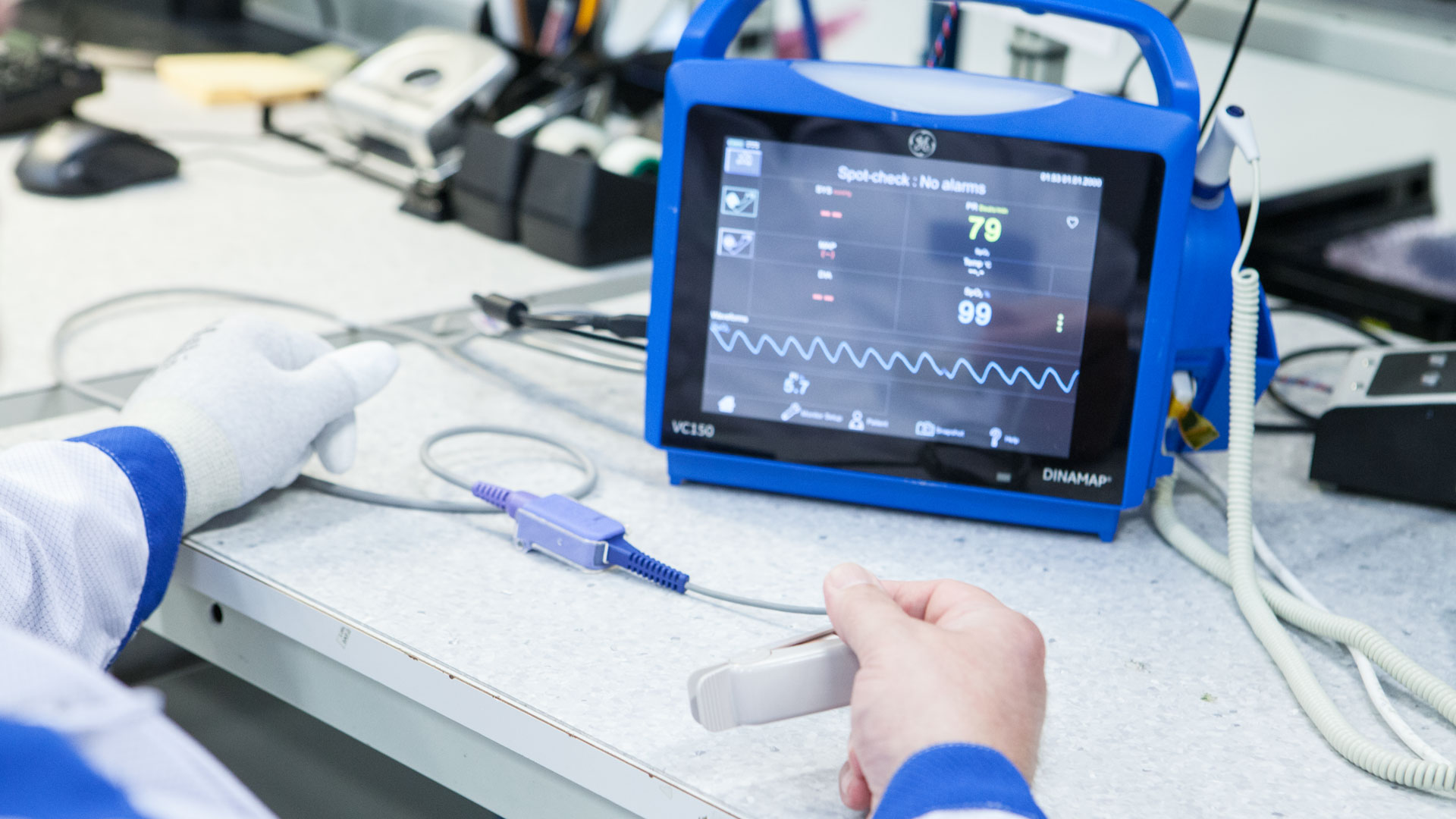
Healthcare technology is one of the largest high-tech export segments of Finnish industry, and the industry continues to grow strongly. According to the latest export report published by Healthtech...
Recent Posts
Human resource management has a direct impact on the company’s performance and the quality of the daily work life. HRM objectives are also highly skilled and motivated employees, who are committed to...
Innokas Medical will participate in the HIMSS Europe & Health 2.0 -event to be held in Helsinki, Finland, on June 11th to 13th. The objective is to bring together healthcare professionals and IT...
We’re living in an amazing world where the new technologies and the science itself enable new kinds of miracles happen in saving peoples’ lives and in improving their well-being. In recent years, one...
Innokas Medical and its personnel will participate in the Cycling Competition (Kilometrikisa in Finnish), which is a playful contest for companies, associations, societies or any other kinds or teams...
Innokas Medical's Helsinki office is reorganizing its facilities and office space during the coming summer to better match our current and future needs.
Innokas Medical and BusinessOulu/OuluHealth are arranging Health Business Breakfast event in Oulu in the middle of June. Welcome to listen, discuss and network with the professionals in the field!
MedTech as a business is changing and getting more diverse. For example, the technical complexity, forms of digitization and regulatory requirements are something that are increasing as we speak. At...
MedTech as business is changing and getting more complex. For example, the technical complexity and regulatory requirements are something that are increasing as we speak. At the same time there is a...