In the field of creating medical devices, there's a crucial meeting point between usability and innovation. The ideas and needs of both creators and users come together to impact the direction of...
Recent Posts
In the ever-evolving landscape of medical device development, usability and innovation intersect significantly. The perspectives of developers and end-users converge to shape the future of medical...
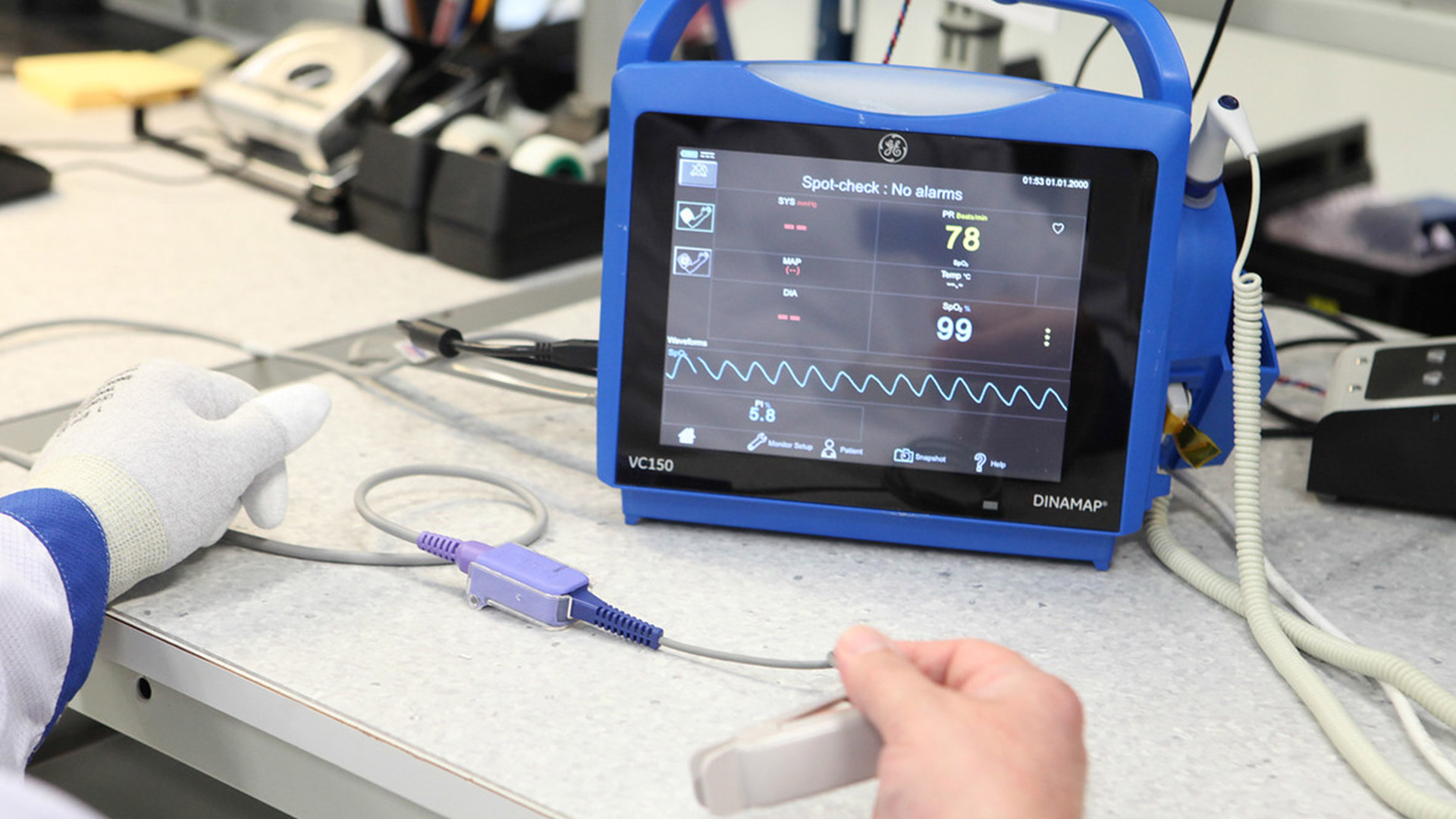
Since 2014, Innokas has been manufacturing VC150 Vital Signs Monitor, a device that has also been designed by our Design Studio. VC150 Vital Signs Monitor is used for sub-acute vitals’ monitoring in...
The Early Warning Score (EWS) is a medical solution taking risk management in healthcare to the next level. Innokas Medical and GE Healthcare have now developed the new version of the software used...