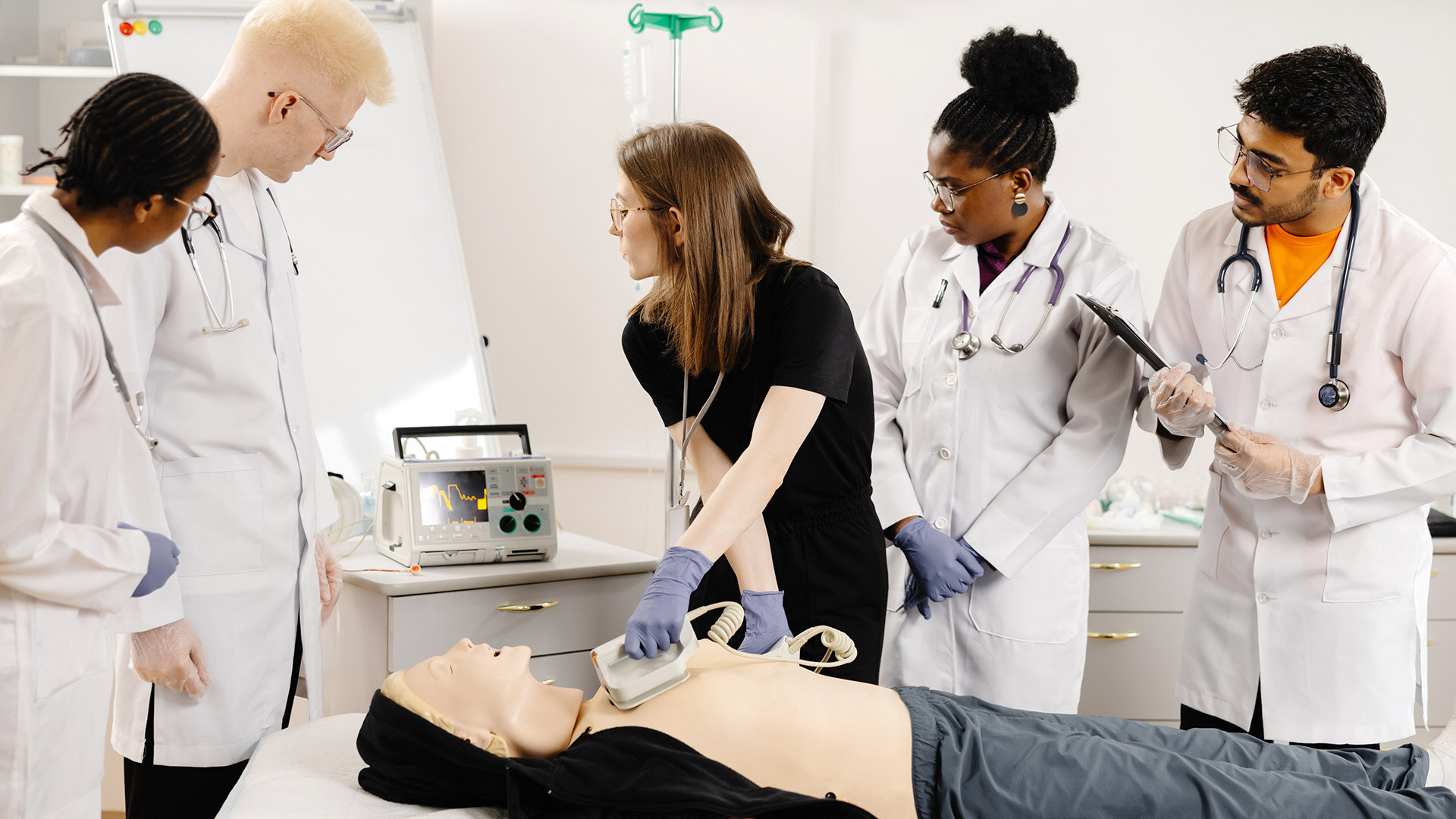
In the field of creating medical devices, there's a crucial meeting point between usability and innovation. The ideas and needs of both creators and users come together to impact the direction of...
Recent Posts
When your project needs medical software proficiency, the first step is assessing needed technical and regulatory expertise. Anyone can access the list of regulations, but their practical application...
In the ever-evolving landscape of medical device development, usability and innovation intersect significantly. The perspectives of developers and end-users converge to shape the future of medical...
In the constantly evolving landscape of technology design and development, efficient collaboration can be the key to streamlined project timelines and short time-to-market goals. Collaboration with...
Sometimes an everyday wellness device might become not just a companion in your journey to better health, but a powerful medical tool that empowers you to monitor, manage, and even predict your...
As of right now, the changes brought on by MDR have quickly heightened the need for clinical evaluation in the medical technology field. During the directive, the approach to clinical evaluation was...
Innokas Medical is present at the Health Valley Event (HVE) that will take place on March 30 with the theme of affordable and accessible healthcare supported by technological innovations. Make sure...
Ever since the European Commission published the new regulations for medical and in-vitro diagnostic devices, there have been serious concerns regarding the availability of medical devices and how...
Innokas Medical launched a service called MDR Fast Track earlier this year. The service enables MedTech companies to enter markets faster with reasonable effort and acceptable risk by outsourcing the...