Complex product development projects are dynamic and require adaptability to maintain control over timelines and budgets while building the winning product. Teaming up with an experienced partner...
Recent Posts
Sustainable product design is increasingly expected aspect in product development. Stricter environmental regulations, consumer demand for eco-friendly products, and investor support for sustainably...
Product developers face a dynamic landscape of shifting priorities, demanding stakeholders, and evolving technologies. In this environment, any of multiple approaches can lead to project success. The...
What happens when you challenge random teams with no prior connection to your organization to design innovative circular design services, and give them two days’ time to do it?
In the constantly evolving landscape of technology design and development, efficient collaboration can be the key to streamlined project timelines and short time-to-market goals. Collaboration with...
Even though material costs have slowly improved following the start of the pandemic, material availability still poses challenges and has introduced disruptions to various product development...
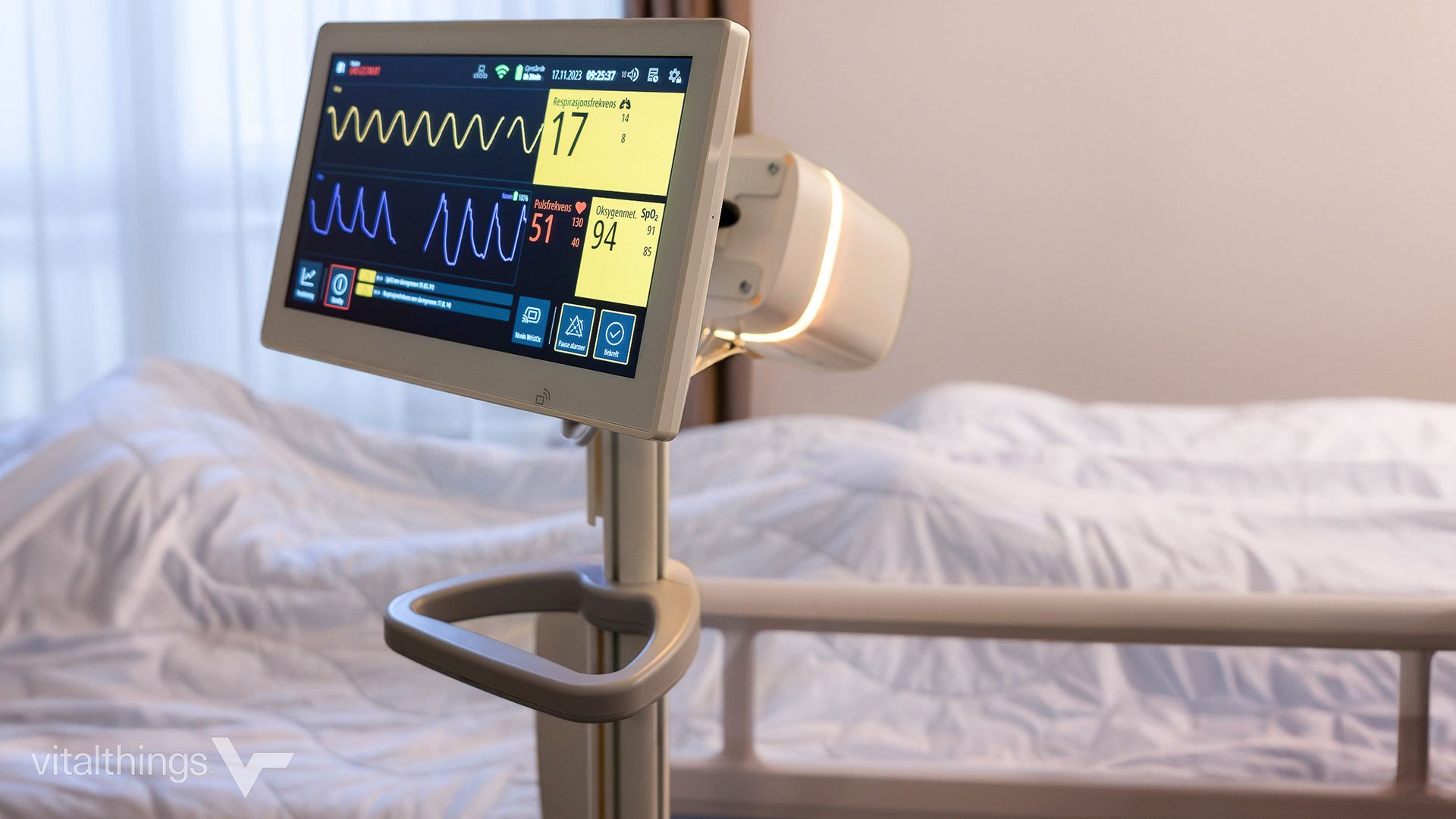
The difference between wellness devices and medical devices lies deeply in the intended use outlined in product definition. That factor often determines how much further product development needs to...
In the dynamic landscape of product development, especially within industries like medical technology, efficiency is key. Utilization of in-house testing during the development has been proven to...
There is an endless potential in utilizing low temperatures to achieve impressive technological feats, such as superconductivity, preserving biological material, and space exploration. Why is it not...