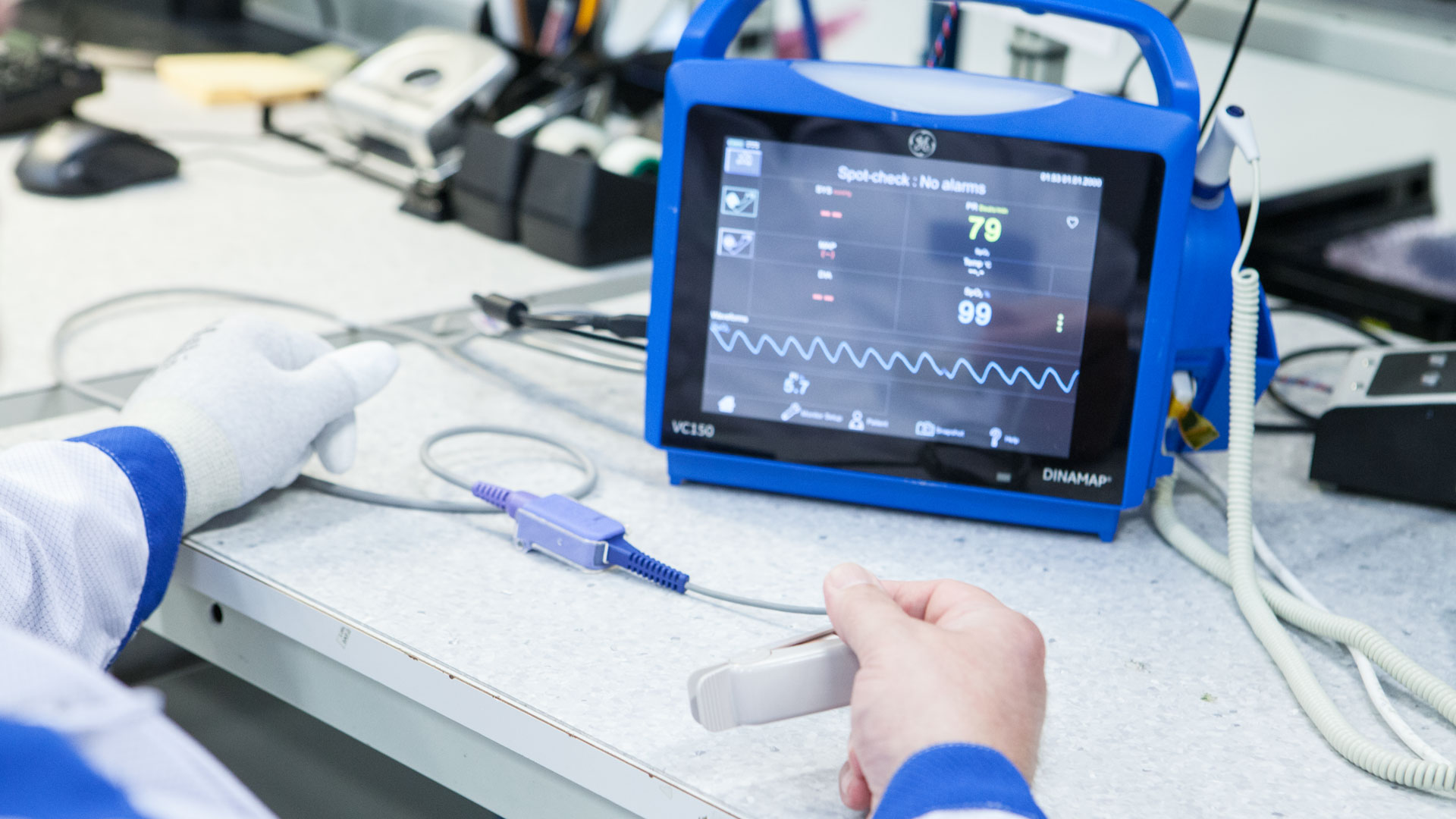
Innokas Medical launches free webinar in series around the topic “Postponed MDR – how to get the best out of upcoming 12 months.” In the first webinar we will discuss about the Clinical Evaluation of...
Recent Posts
Innokas Medical has been invited to Health Tuesday -event organized by Business Finland, to tell our story how we have implemented the essential European regulatory requirements. Welcome to listen,...
Warm thanks for the pleasant cooperation this year. We wish you all a happy Easter and all the best for the coming spring season! 🐥☀️- Innokas Medical
The objective of Finnish-based IVD company Aidian is to promote efficient and fluent healthcare by developing and manufacturing reliable, fast and easy to use diagnostic tests for point of care....
SoteDigi, who develops new kinds of digital solutions for patient- and health care, selected partner companies for further development of its Omaolo.fi -service solution in the beginning of this...
According to the latest export report published by Healthtech Finland, the exports of the Finnish health technology reached again a new record in 2019. Thus, Finnish health technology has kept up...
Many medical device design or manufacturing companies may find it difficult to allocate enough time and resources to master the regulatory compliance of medical device, and to compile, maintain, and...