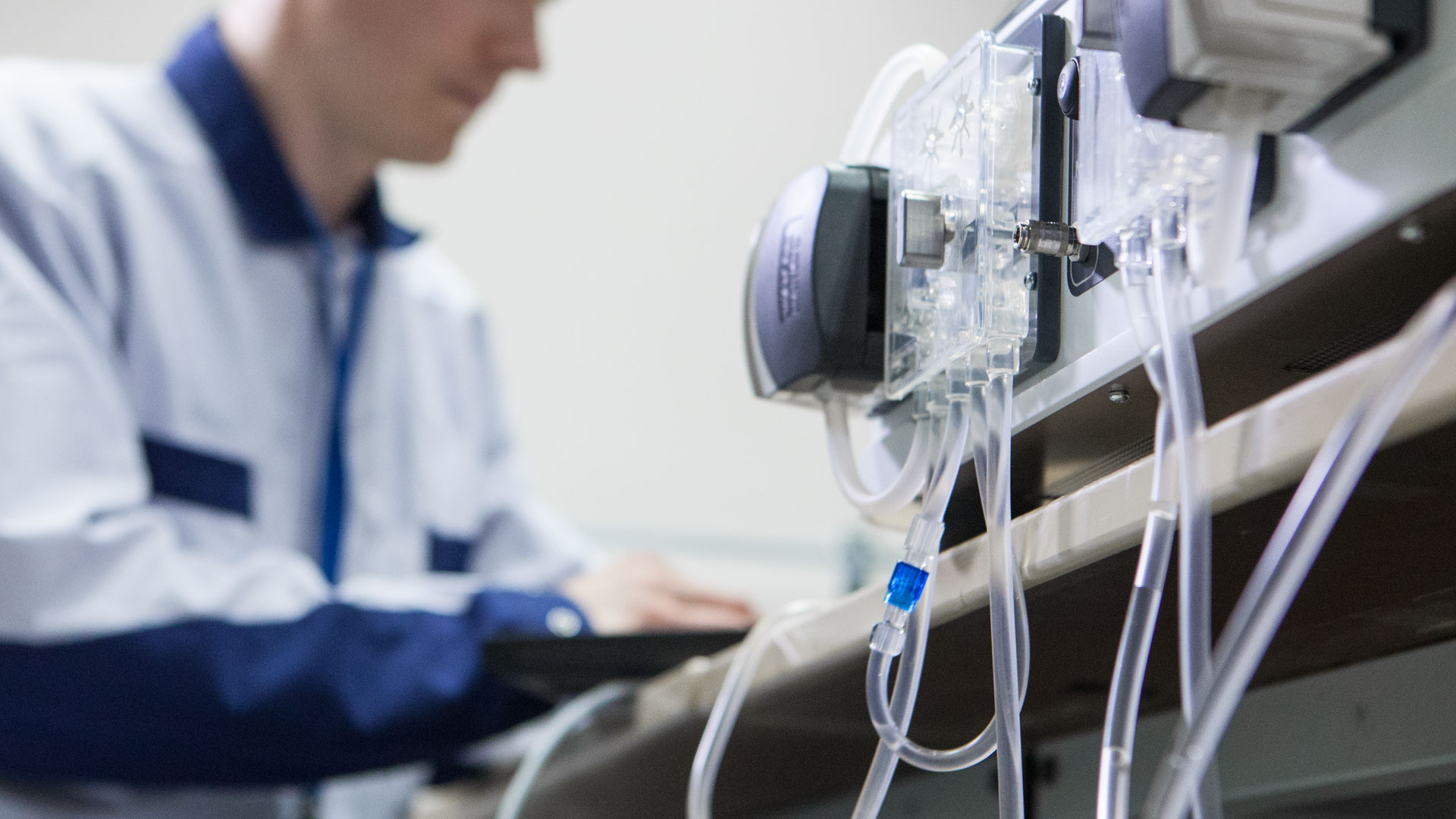
A poorly managed medical device NPI (New Product Introduction) is an expensive one, so being prepared is a good start. Are you looking to improve your existing device with a new manufacturing...
Recent Posts
Whether there is a design of electromechanical device that is still in its working phases or a product that already has a history of being produced at another location, ramp-up can be a challenging...
When creating a new medical device to the market, there’s much more that goes into designing, developing and introducing it to the market. To be able to enter the certain markets, the development and...
New Product Introduction (NPI) and Design Transfer are both important parts of the whole life cycle of medical product from idea to market launch. Successful prototype series and professional...
The cooperation between Innokas Medical and Monidor Ltd. has developed to the next level during this year. The first steps of the cooperation were took already in 2015 when Innokas was offering QA...
Innokas Medical and the Norwegian company Luzmon Medical AS have recently signed a manufacturing contract for the Luzmon’s cTEMS and cTENS system including some NPI and sourcing work. The agreement...
Innokas Medical and Icare Finland have recently signed a manufacturing agreement. The contract covers the manufacturing of Icare® HOME tonometer at Innokas Kempele factory. The actual work has...
The Carescape VC150 patient monitor, developed in cooperation between Innokas Medical and GE Healthcare, has reached a significant milestone as the monitor has been released for sale. In addition to...
Innokas Medical and the Swedish company Bonvisi AB have recently signed an R&D agreement, and shall soon sign a manufacturing contract for the Bonvisi ENDO Pump system – ENDO P® – endoscopic surgery...