The new Medical Device Regulations (2017/745/EU) (MDR), published in May 2017, will replace the existing Medical Devices Directive (93/42/EEC) (MDD) and the Active Implantable Medical Devices...
Recent Posts
Innokas Medical will again participate in Digital Health Nordic -event, which will be held in Helsinki in the beginning of February. The event provides a unique look at the role of technology in...
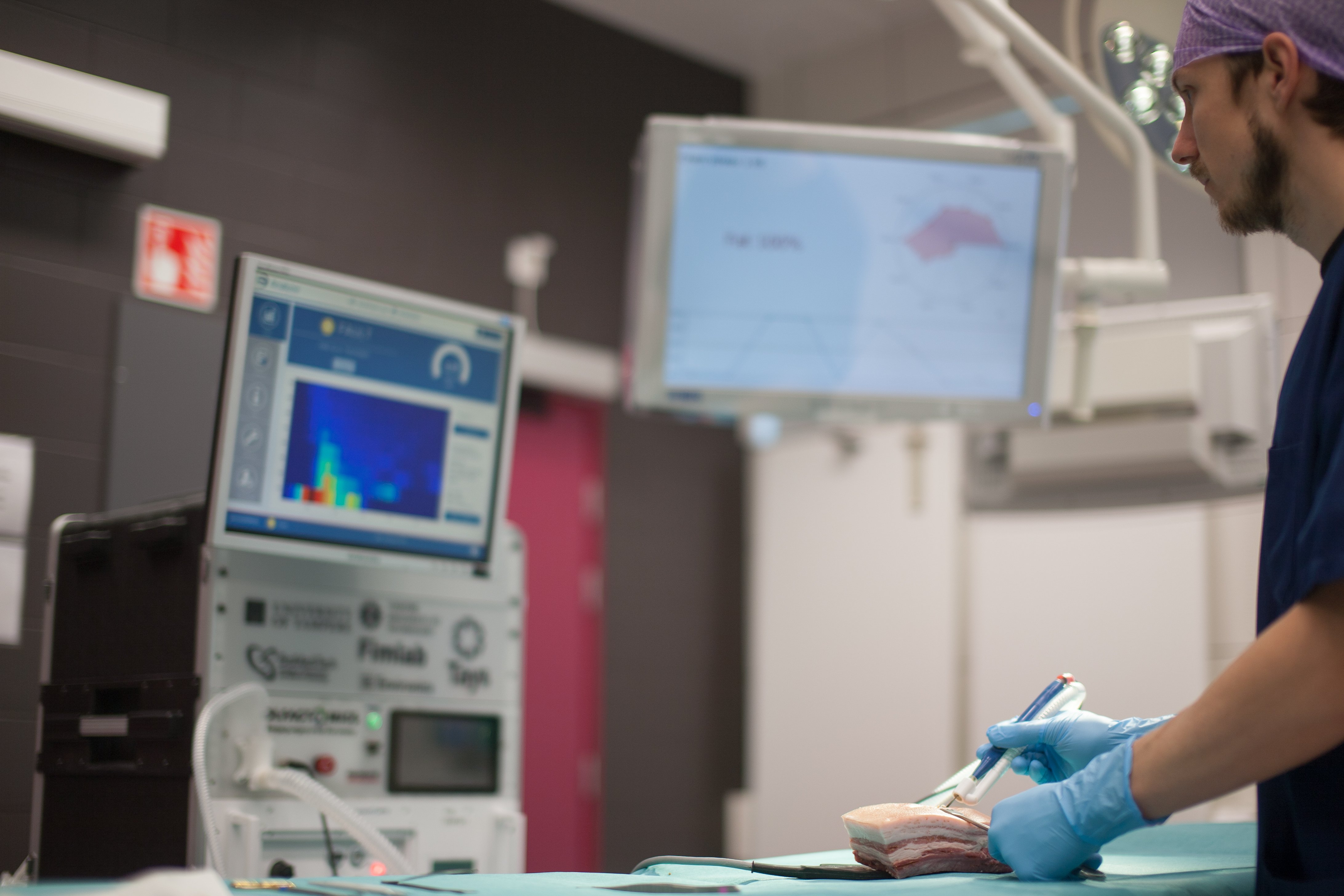
Olfactomics Ltd, with whom Innokas Medical started a cooperation in 2018, is a Finnish startup company that develops a smart surgical blade capable of detecting cancer during surgery. The device is...
Innokas Medical will be visiting Arab Health next week. Arab Health is the largest medical exhibition and conference in the Middle East. It will be arranged in Dubai from 28th to 31st January.
New year and new beginnings – but first it’s time for 2018 year-end list! We took a look at which blog posts on Innokas Medical’s Business Blog were the most popular ones; so here are our top 10 blog...
The year 2018 draws to a close and the Christmas Season is just about to begin. Warm thanks for the pleasant cooperation this year. We wish you all a peaceful Christmas time and all the best for the...
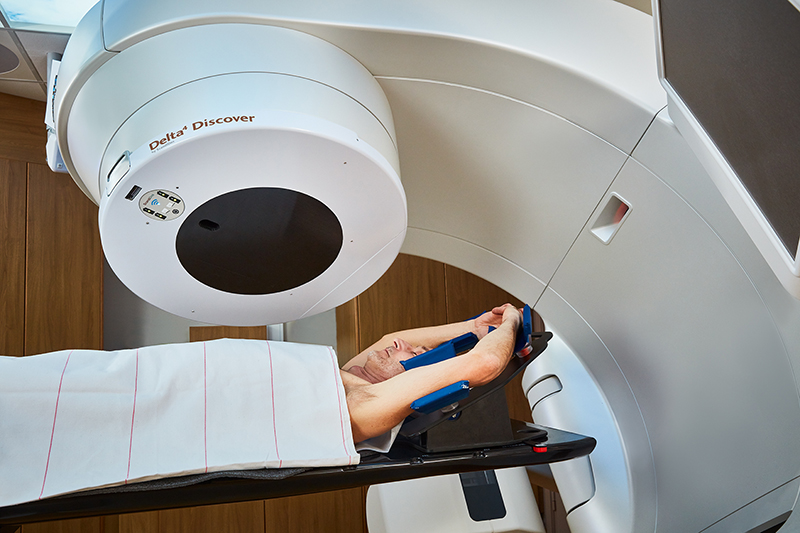
Innokas Medical has entered into manufacturing collaboration with Swedish medical technology company ScandiDos AB. This collaboration brings yet another Swedish customer to Innokas, providing...
Finding answers? Welcome! - This is an unofficial guidance which is intended to assist medical device OEMs by sharing some tips on how to prepare to the new European Medical Device Regulations,...
Innokas Medical supports children and their families spending the Christmas at hospital this year. The company organized Christmas charity campaign with its personnel in favor of Ronald McDonald...