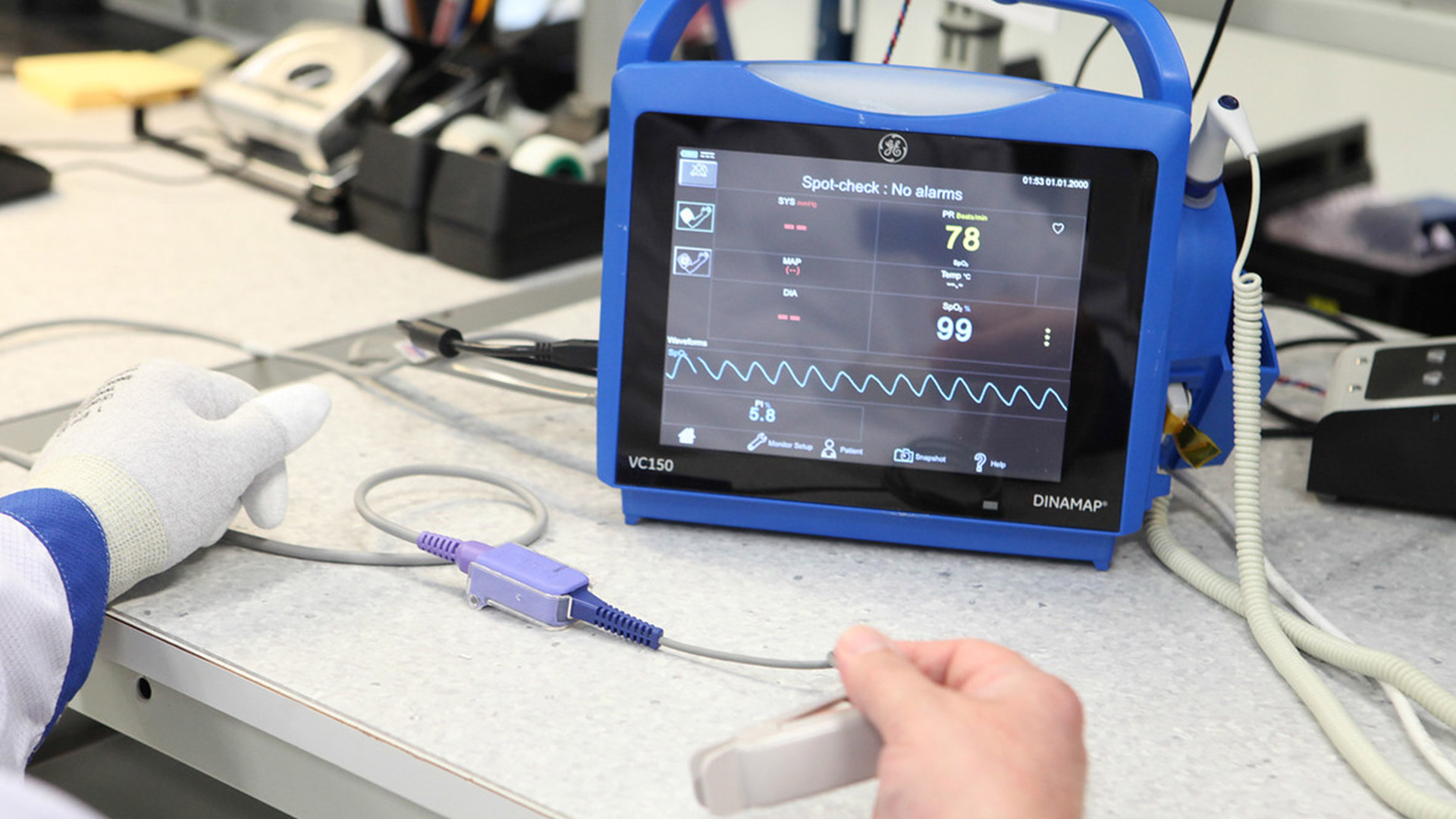
Since 2014, Innokas has been manufacturing VC150 Vital Signs Monitor, a device that has also been designed by our Design Studio. VC150 Vital Signs Monitor is used for sub-acute vitals’ monitoring in...
Recent Posts
Manufacturers who have devices CE Marked to the directives MDD, AIMDD or IVDD (i.e., legacy devices) have deadlines for regulatory compliance if they want to keep their devices on the European...
We are proud to announce that Innokas Medical is taking part in Circular Design training program.
In 2022, Technology Industries Finland challenged companies to hire experts from among international students. The goal of the campaign was to give the students Finnish working life experience,...
The new MDR regulation has expanded the need for clinical evaluation in companies designing medical devices. The interview for my master's thesis highlighted the requirements set by the new...