Greetings from the “Husky Company” you might have heard of at DMEA 2025. Our eventgoers Visa Poikela and Päivi Leppänen discussed the event's quality and networking opportunities and highlighted the...
Recent Posts
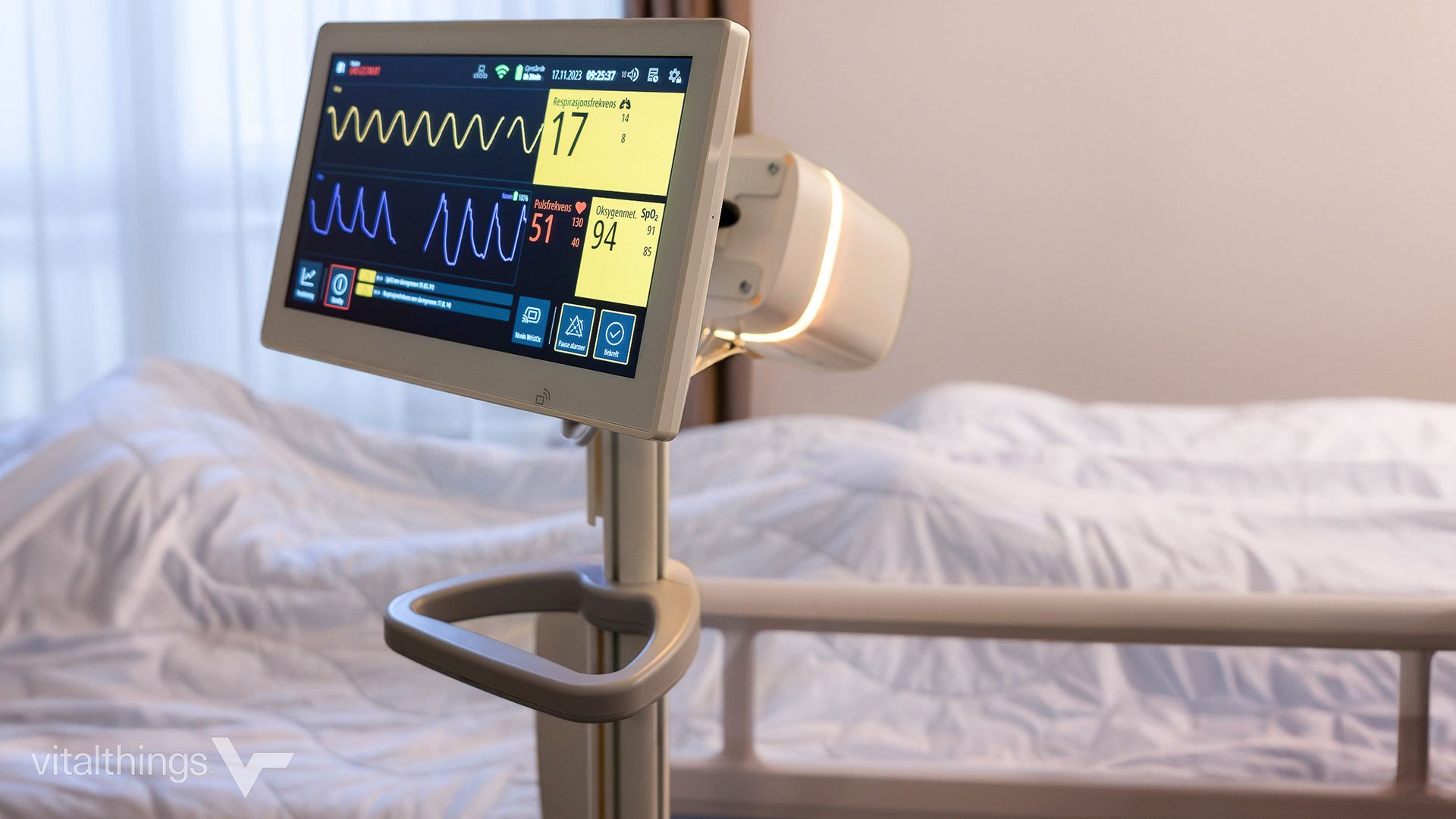
Innokas was chosen by Orla DTx to design and develop a software library for asthma-related calculations to ensure their product meets MDR requirements as a Class IIa medical device. The collaboration...
Complex product development projects are dynamic and require adaptability to maintain control over timelines and budgets while building the winning product. Teaming up with an experienced partner...