Do you think of quality management as only one part of the progress of bringing your software device to the market? Preferably one that can be checked out as a last step before your software device...
Recent Posts
To succeed in taking a medical device product from idea to the market, both the design and development flow, as well as the regulatory flow, need to be considered. As the inventor or manufacturer of...
We all want to see into the future; would you like to predict what kind of problems you could run into during electromechanical device manufacturing process well before those problems happen? While...
Innokas Medical is present at the Health Valley Event (HVE) that will take place on March 30 with the theme of affordable and accessible healthcare supported by technological innovations. Make sure...
Jelena Gladõseva is nearing her third-year milestone working at Innokas Medical’s Tallinn location. The location is a volume-producing factory that has been in operation for over ten years. Jelena...
Whether there is a design of electromechanical device that is still in its working phases or a product that already has a history of being produced at another location, ramp-up can be a challenging...
As our team took part in Arab Health in Dubai, they were keeping their ears and eyes open for new trends in the industry. The special interest was to see whether issues of sustainability are...
Walking on the streets of almost any city, we can see that there are quite a bunch of building sites, cranes, and upcoming wonderful colors on the walls of new hospitals enabling more modern...
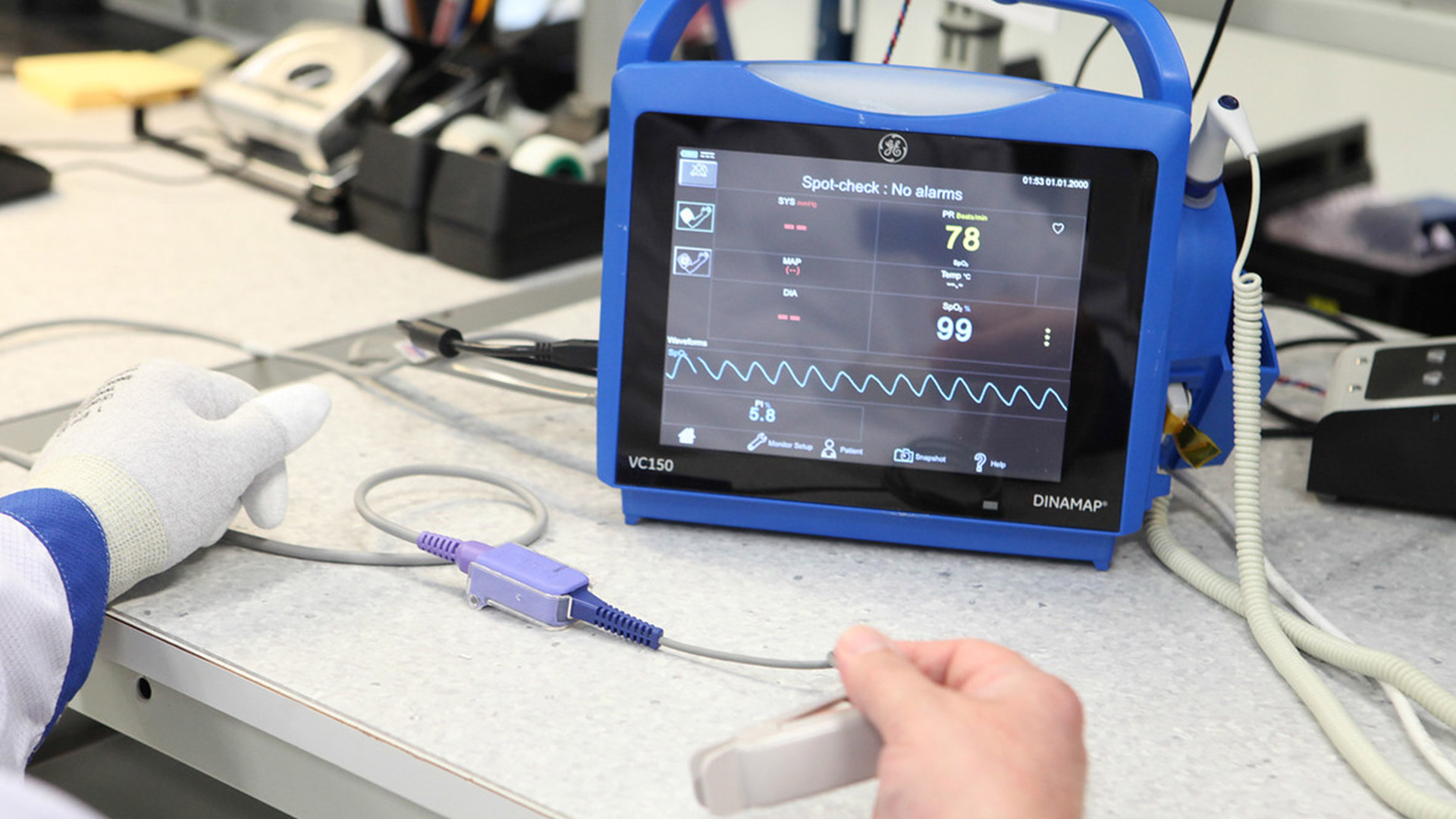
Since 2014, Innokas has been manufacturing VC150 Vital Signs Monitor, a device that has also been designed by our Design Studio. VC150 Vital Signs Monitor is used for sub-acute vitals’ monitoring in...