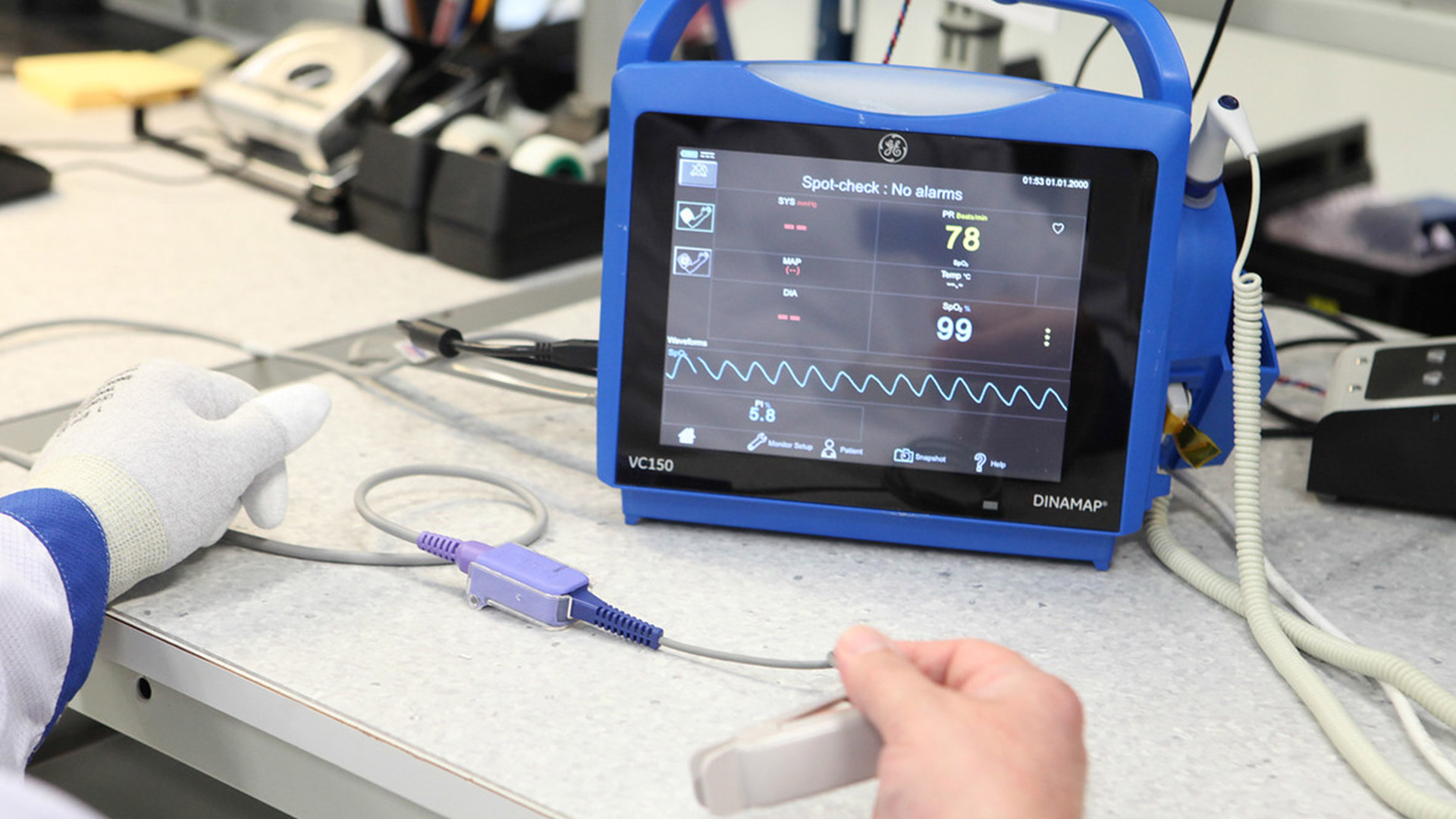
Since 2014, Innokas has been manufacturing VC150 Vital Signs Monitor, a device that has also been designed by our Design Studio. VC150 Vital Signs Monitor is used for sub-acute vitals’ monitoring in a hospital environment. The monitor can measure oxygen saturation, respiration rate, heart rate, blood pressure, and body temperature. It can be moved easily around the hospital connected to hospital’s WI-FI network.
Innokas acts as a responsible manufacturer of VC150 Vital Signs Monitor maintaining device software and hardware. Innokas has taken care of the regulatory approvals of the device in various market areas including USA and EU and Middle East. VC150 Vital Sign Monitors are currently used in over 30 different countries around the globe.
What to consider when designing a successful health monitoring product
A monitor design needs to be safe, desirable, robust, easy to use and cost-efficient. Less is usually more when these requirements are fulfilled. If technical details are decided without keeping the manufacturability in mind, no factory can efficiently produce the product without issues. To ensure manufacturability in design, it is important to select the manufacturing partner and involve them in the project as early as possible.
There are also a wide variety of different regulations and requirements which need to be taken into consideration. For instance, cyber security is more and more critical with connected devices. Continuous attention and maintenance are required to stay safe from cunning hackers. Regulations tend to vary in different market areas.
Usability of your medical device can make or break your business. Usability is a combination of user interfaces, user experience and usability processes. Healthcare professionals need many functionalities from devices, to guarantee efficient care and operability in the hospital environment. The user interface is at the center of usability. However, usability doesn’t only mean that a device is easy to use. Ergonomics and many other factors need to be naturally fine-tuned to a proper level, and the safety of the patient and the user always comes first.
Choosing Innokas as a partner
There are various advantages to having a contract design and contract manufacturing partner. You do not have to hire and then possibly lay off design resources for and after your unique medical device development project. You also do not have to bind your own capital into production.
We at Innokas are specialized in demanding contract design and contract manufacturing of electromechanical medical devices. Innokas has almost 30 years’ experience in challenging medical device design and manufacturing. Innokas manufactures in-house PCB assemblies, assembles medical devices, and tests those devices before delivery to the customer. You can check out the reference story here.
Our competent staff ensures cost efficiency in design and in manufacturing processes. Since Innokas is making both design and manufacturing, manufacturability is built into product designs. Design for Excellence methods are applied without discontinuations. We can design a product for you, manufacture it, and even act as a responsible manufacturer. Our comprehensive service portfolio enables you to focus on the sales and marketing of your medical device.
Why partner with a subcontractor in Europe?
Lately, there have been various abnormalities shaking the global supply chain. Uncertainty in the political situation, trade barriers, natural disasters and frequent global epidemics require great risk aversion strategies. Subcontracting and splitting manufacturing to different locations might make a positive difference in a case of an unexpected supply chain hiccup.
If you are a European company selling in Europe, geological and cultural proximity might reduce delivery times and ease cooperation. If you are an Asian company exporting to EU or to USA, manufacturing in Europe might nowadays be a cost-efficient alternative because of lower or totally missing customs tariffs and shorter delivery distances and times. For more information, please