Here is a recipe on how to push through the MDR process in the shortest possible time. Based on my recent panel presentation at the MedFIT 2024, I have put together some key tips and ideas on what to...
Recent Posts
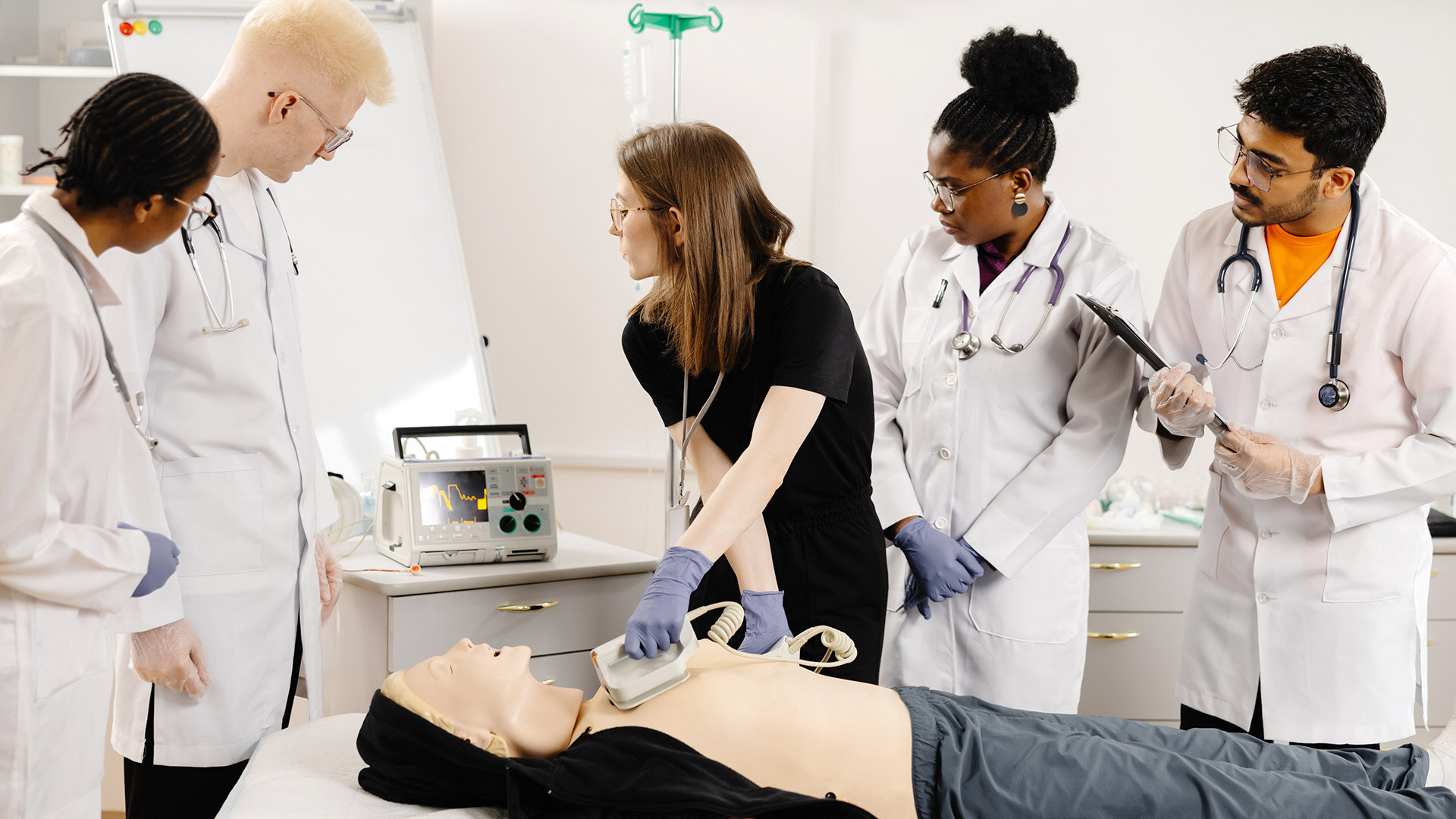
In the field of creating medical devices, there's a crucial meeting point between usability and innovation. The ideas and needs of both creators and users come together to impact the direction of...
Returning to the Radical Health conference for the second year in row, we already knew a little of what to expect. Innokas was already quite well-known among the participants, which made initiating...
We all know that our world continues to become more digitalized. But did you also know that this is happening alongside a shift in our healthcare? Patients who used to be more passive in receiving...
In the ever-evolving landscape of medical device development, usability and innovation intersect significantly. The perspectives of developers and end-users converge to shape the future of medical...
Report written by Innokas experts and event goers Visa Poikela & Päivi Leppänen
Been away from the wet lab for more than 15 years, attending Analytica was an eye-opener. As someone stepping back...
There are two distinct company types with different starting points when they begin searching for suitable European contract manufacturer. The first is a new start-up in search of their first...
Report written by Innokas experts and event goers Päivi Leppänen & Visa Poikela
Contact information available at the end of the post
Sustainability in Medtech was a hot topic. It's clear that the...
If you were asked to picture what good teamwork looks like, how would you picture it? How about a dog sled, traveling with efficient pace and agility through harsh snowy terrain.