In the field of creating medical devices, there's a crucial meeting point between usability and innovation. The ideas and needs of both creators and users come together to impact the direction of medical technology. In the second installment of this blog series, we delve into the thoughts of another expert from Innokas QA&RA, focusing on usability engineering. Here, we uncover the importance of this often-neglected aspect of product development. QA&RA specialist Minna Eskola shares her experiences and insights on this journey.
My interest in usability was piqued at Helsinki University of Technology (today known as Aalto University) and thanks to my internship, I started to learn about the regulated usability engineering of medical devices. Over the years, I have had the privilege to do usability engineering work in different kinds of projects. Here are some experiences from the past.
First part of this blog series: Medical device usability insights from clinical affairs specialist
Understanding different devices and different usability requirements
Innokas partners with a diverse array of customers, but when a new project starts, it’s the start of a new world. The intended use and device details are likely different from my previous experience. However, past projects and insights from other devices help define the usability engineering framework, scale the workload, and determine necessary actions.
As usability of a medical device is not the same as usability of a consumer device, some customers become aware of specific usability engineering requirements on short notice and ask for support in implementing them. Sometimes the wake-up call comes so late that the device is basically designed and developed. Even in this kind of situation, Innokas QA&RA team can review the technical documentation from a safety viewpoint and fill in the gaps in the usability engineering process for the customer.
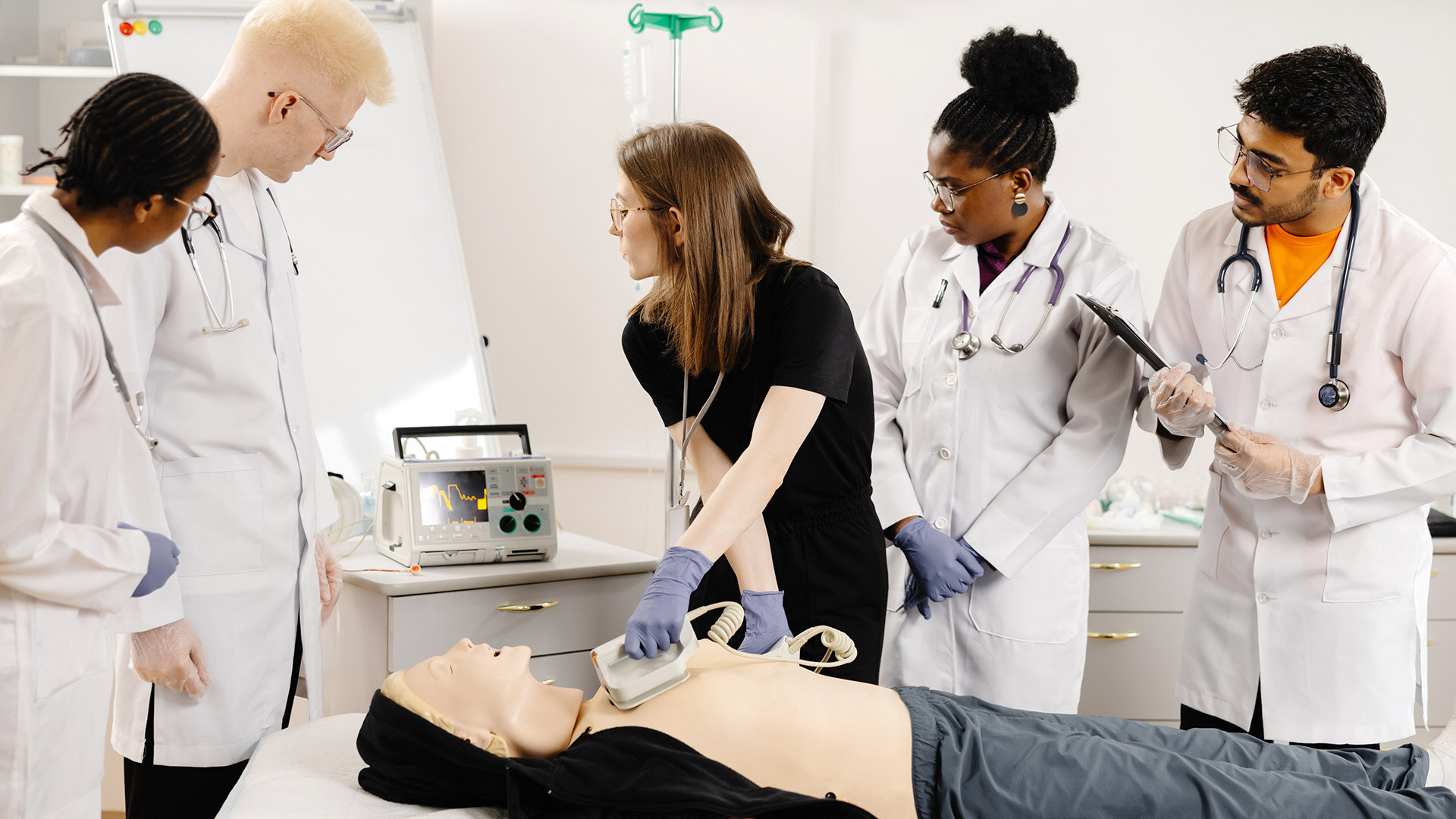
Understanding practical use environments for usability testing
We have implemented usability engineering tasks, e.g., in an office space with a simulated operating room, in a more mature version of simulated operating room in HUS Testbed in Helsinki, and in a similar setting in OYS TestLab in Oulu. When Covid-19 started closing the world, we executed multiple rounds of formative evaluation over Teams with a customer developing a patient monitoring system. It wasn't optimal, but it was feasible and provided the necessary input to advance device development.
In some projects, the medical device is meant for use by lay users in home healthcare settings. I've conducted evaluations at my home and visited intended users in theirs. I have participated in usability engineering activities in the US, Italy, France, Norway, and various locations in Finland.
Understanding how formative evaluation allows direct contacts with intended users during testing phases
Formative evaluation (iterative usability verification during medical device development) is my personal favorite part of the usability engineering process. This evaluation can be implemented in many ways, can be very informal if wanted, and allows contact with intended users early on during the design and development. It is rewarding to meet the evaluation participants and see and hear how they interact with prototypes. It is especially gratifying when our customers, typically the owners of the design, see their device in the hands of the users and gain understanding to who and which purpose they design their device. It's satisfying to confirm correct design decisions, yet it's also valuable to recognize potential user variations, unforeseen risks, or differing user expectations that may arise during device use.
Experienced partner teaches understanding
Some customers begin seeing usability so important that they want to have in-house knowledge and expertise to handle usability engineering processes themselves. It makes me happy to see them attend training, create required documentation, practice in the role of evaluation moderator, perform evaluations and achieve good results. We at Innokas provide support as long as necessary and transition away when the customer can operate independently.
I do not have a medical background, but I enjoy learning about our customers’ devices, about the environment they are used in, and about the professionals or lay people who will be using the devices. Over the years, healthcare professionals have given me a white jacket to wear and allowed me to stand in the corner, lurk behind their backs, peak from the sterile area, and ask multiple questions while observing them work and perform different medical procedures.
During these contacts with medical device users, I have been impressed by the expertise, determination, calm know-how, teamwork, and willingness to share knowledge and improve means to treat patients. As a Usability Specialist, I am proud to work towards bringing safe medical devices for professional and lay users.
Innokas serves as a reliable ally in navigating usability engineering within medical device creation. Whether you're in the initial stages of brainstorming or refining your product for market readiness, Innokas provides a range of specialized services to tackle your usability challenges. From expert advice to practical assistance, our devoted team is determined to assist you in realizing your goal of introducing groundbreaking medical innovations. Connect with us by filling out a contact form via the link below, and we'll promptly respond to your inquiry!
Author

Minna Eskola
QA&RA specialist
minna.eskola@innokas.eu