Manufacturers who have devices CE Marked to the directives MDD, AIMDD or IVDD (i.e., legacy devices) have deadlines for regulatory compliance if they want to keep their devices on the European...
Recent Posts
Innokas Medical launched a service called MDR Fast Track earlier this year. The service enables MedTech companies to enter markets faster with reasonable effort and acceptable risk by outsourcing the...
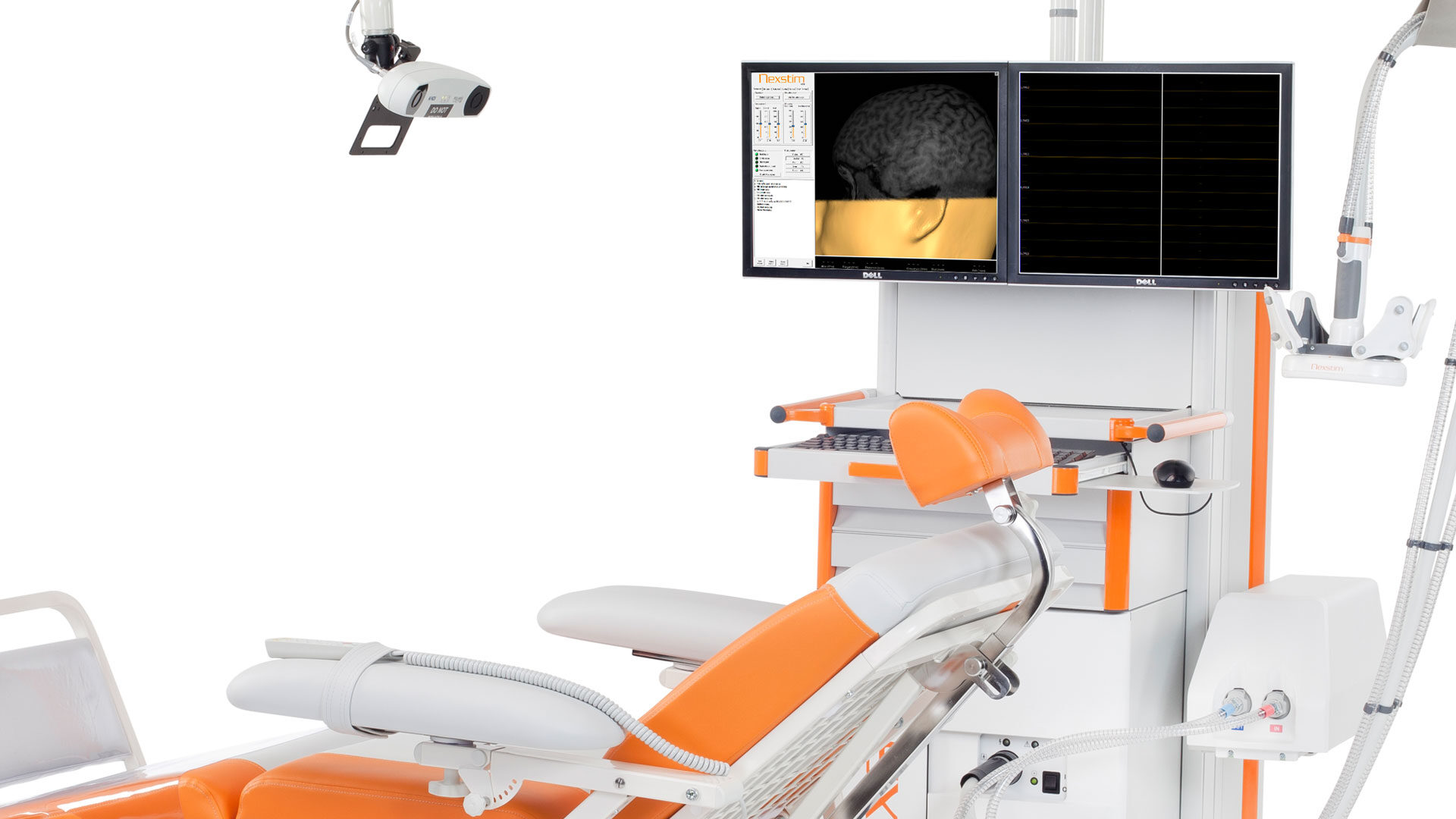
Nexstim Plc. develops and manufactures NBS equipment for demanding brain diagnostics. The NBS (Navigated Brain Stimulation) System combines 3D brain mapping, stimulation coil positioning, and...
UKK Terveyspalvelut Oy, whose headquarters are located in Tampere Finland, is working with an important matter: together with its main owner, the UKK Institute, the company promotes the...