The saying goes “find a job you love, and you will never have to work a day in your life”. Turning a beloved hobby into your career is in the hearts of many people; supporting yourself by expressing...
Recent Posts
Efficient cooperation between expert partners and well-fitted practices and tools can significantly impact the time to market, reduce costs and simplify the product development of complex solutions.
Product developers face a dynamic landscape of shifting priorities, demanding stakeholders, and evolving technologies. In this environment, any of multiple approaches can lead to project success. The...
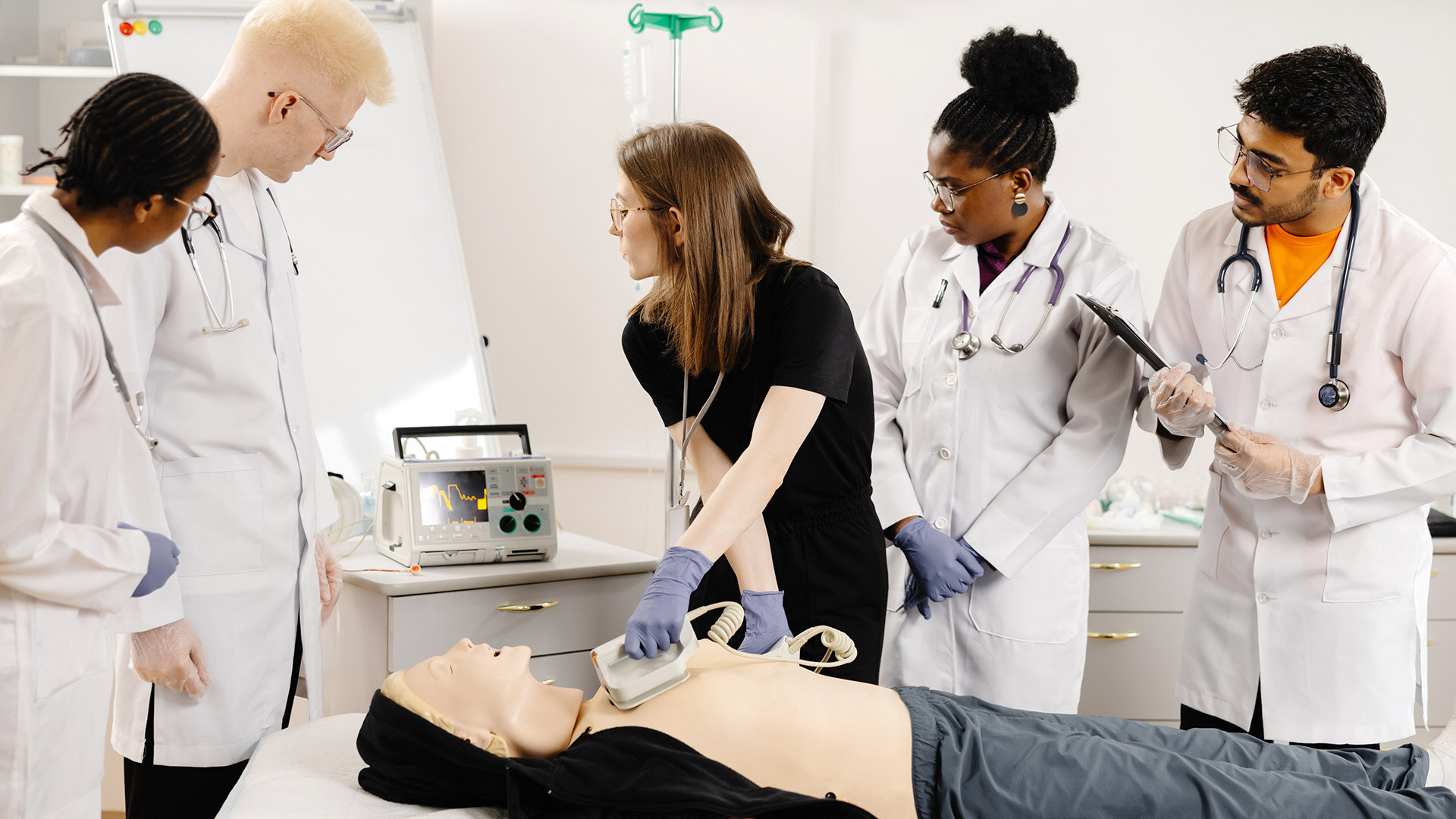
In the field of creating medical devices, there's a crucial meeting point between usability and innovation. The ideas and needs of both creators and users come together to impact the direction of...
Returning to the Radical Health conference for the second year in row, we already knew a little of what to expect. Innokas was already quite well-known among the participants, which made initiating...
What happens when you challenge random teams with no prior connection to your organization to design innovative circular design services, and give them two days’ time to do it?
We all know that our world continues to become more digitalized. But did you also know that this is happening alongside a shift in our healthcare? Patients who used to be more passive in receiving...
When your project needs medical software proficiency, the first step is assessing needed technical and regulatory expertise. Anyone can access the list of regulations, but their practical application...
It’s a remarkable day for Innokas; our first ever sustainability report has been made public!