High quality combined with cost-effective production can be achieved through optimized manufacturability. This requires a wide range of expertise as well as strategic capabilities.
Recent Posts
As Finnish and global governments and various medias have communicated, COVID-19 disease, also known as coronavirus, has spread worldwide as a major health threat. In addition, it has already caused...
Especially now, due to COVID-19, the global demand is high for ventilators and other critical care products. At Innokas Medical, we have readiness and capacity to start the production of critical...
Innokas Medical will participate in Medico Bazar 2020 -event which will be held in Lyngby, Denmark (in Technical University Library) on 12th March. Medico Bazar displays the newest devices, projects...
When creating a new medical device to the market, there’s much more that goes into designing, developing and introducing it to the market. To be able to enter the certain markets, the development and...
Innokas Medical will participate in European Healthtech Investment Forum –eventwhich will be arranged in Helsinki, Finlandon 10th to 11th March 2020. The event brings together leading investors...
Information Driven Care in Response to Healthcare Challenges were discussed in Digital Health Nordic
Innokas Medical participated in Digital Health Nordic 2020 -event together with its sister company Cubist IT AB from Sweden. Innokas and Cubist were the main sponsors of the event. Digital Health...
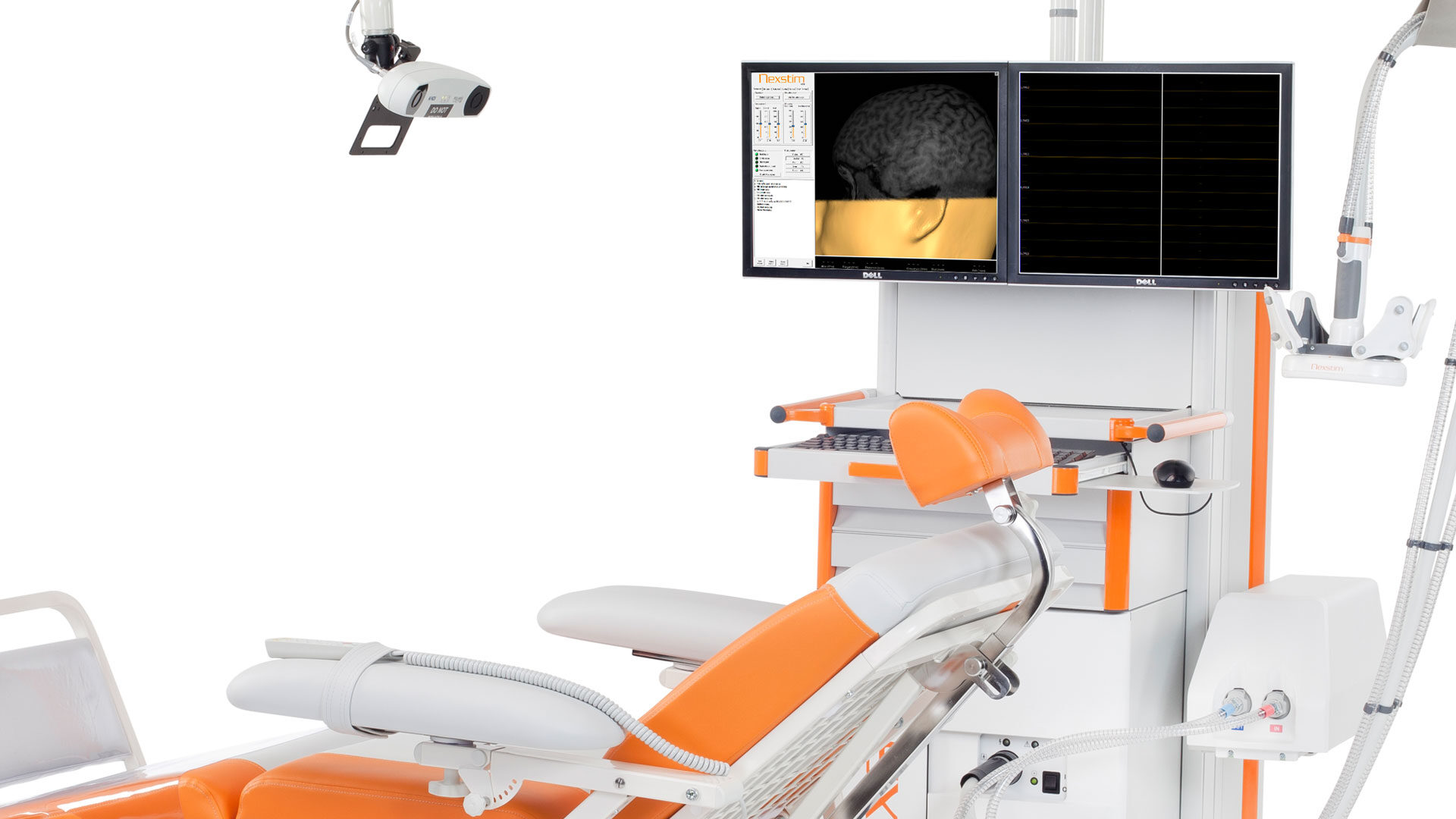
Nexstim Plc. develops and manufactures NBS equipment for demanding brain diagnostics. The NBS (Navigated Brain Stimulation) System combines 3D brain mapping, stimulation coil positioning, and...
Innokas Medical and Tampere HealthHub are arranging Health Business Date -event in Tampere at the end of February. Welcome to listen, discuss and network with the professionals in the field!