Together with our cooperation partner Swedish Medtech we would like to discuss about Clinical Evaluation of Medical Device Software. Thus, we've been invited to hold an online event with Swedish...
Recent Posts
FlowOx 2.0, developed in co-creation between Norwegian company Otivio AS and Innokas Medical, is a clinically promising, patented and cost-efficient home treatment solution for Peripheral Arterial...
Innokas Medical and our partner Biocodex PhaMe have been invited to BioTreffit & Health Tuesday -event organized by Turku Science Park Oy and Business Finland, to discuss about development of...
The Innovation Voucher, offered by Business Finland, is one funding opportunity for Finnish SMEs who seek for purchasing new knowledge and skills for their business. According to Business Finland,...
Innokas Medical will participate in Aalto Talent Expo Online event, which will be organized virtually on 12th to 14th January 2021.
The year 2020 draws to a close and the Christmas Season is just about to begin. Warm thanks for the pleasant cooperation this year. We wish you all a peaceful Christmas time and all the best for the...
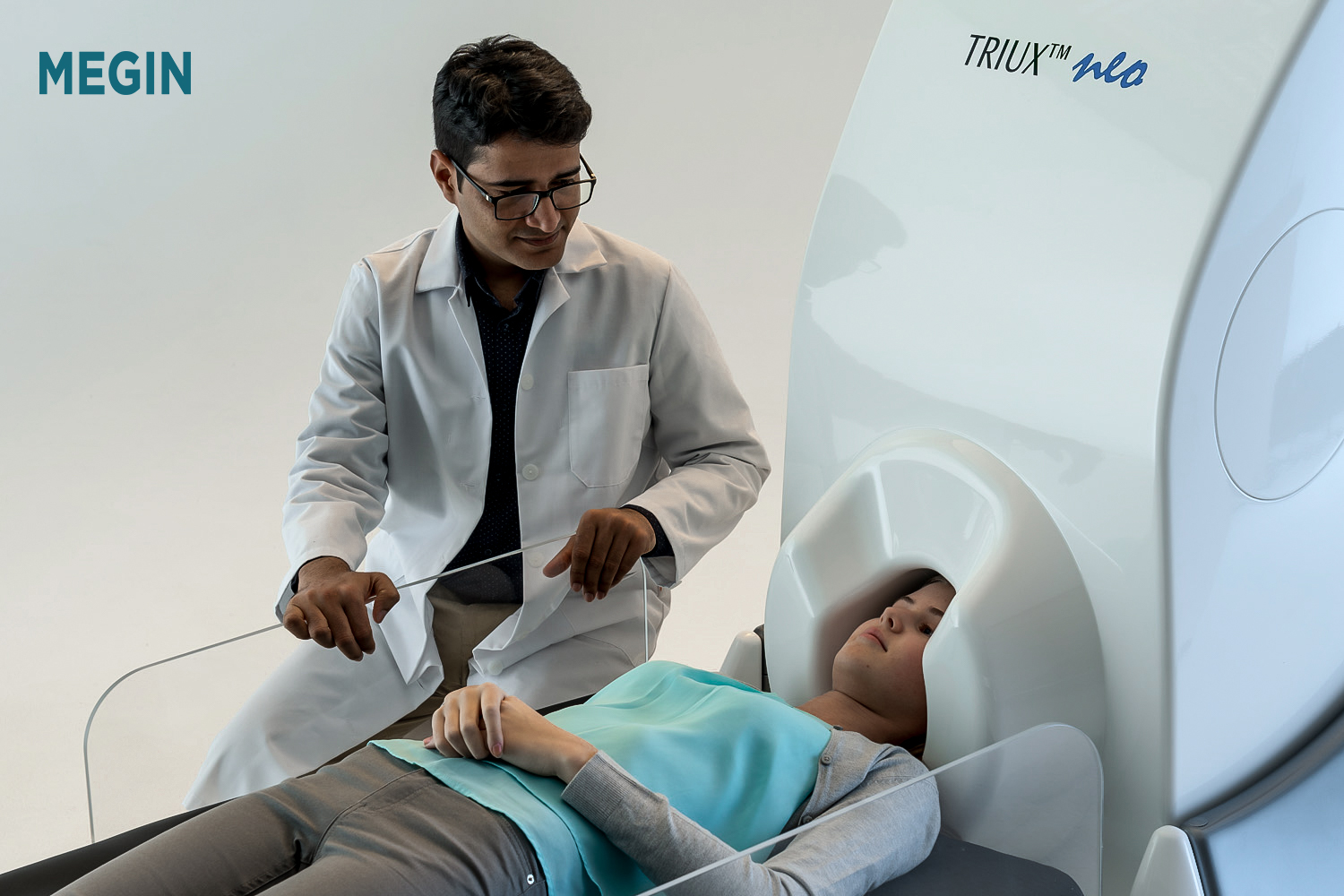
Headquartered in Helsinki, Finland, MEGIN is the global leader for magnetoencephalography (MEG) technology used for their non-invasive functional brain mapping solution. A long-term collaboration...
Innokas Medical, with expertise dedicated to medical devices and software, and Biocodex PhaMe, providing pharmaceutical consultant services, have agreed on a strategic partnership. Through this...
It has been noted that the occurrence of use errors, also called as human error, increases the number and severity of incidents related to the use of medical devices. Use errors typically occur when...