Headquartered in Helsinki, Finland, MEGIN is the global leader for magnetoencephalography (MEG) technology used for their non-invasive functional brain mapping solution. A long-term collaboration between MEGIN and Innokas Medical has established a trusted partnership for both parties.
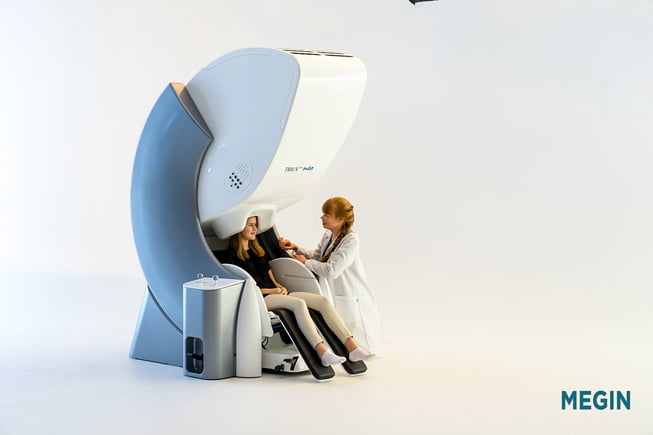
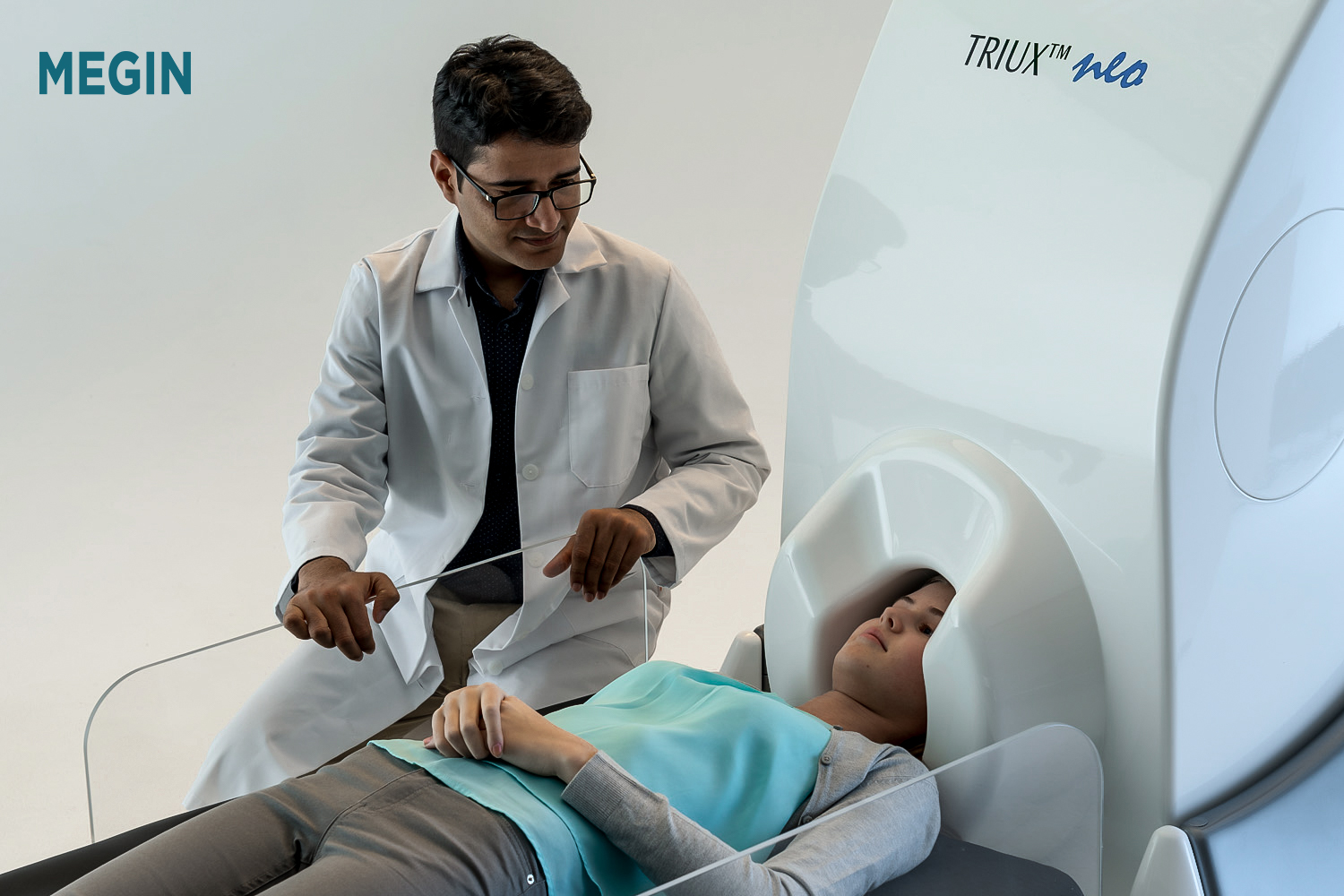
With their functional brain mapping solution, TRIUX™ neo, MEGIN is impacting diagnostics and treatment of neurological diseases with its ability to detect magnetic fields generated in the brain, where information travels through a very complex neural network. When utilizing the information offered by TRIUX™ neo, physicians can provide minimally invasive treatment options that can lead to improved outcomes and minimize the potential for neurological deficits.
“Our solution aims for better diagnostic workup, treatment strategy and intervention of neurological conditions as it supports clinicians with decision-making”, tells John Fulford, Managing Director at MEGIN.
In general, magnetoencephalography (MEG) is a technique for mapping brain activity. Measurement is done by recording magnetic fields generated by electrical currents occurring naturally in the brain.
“Brain functions are based on neural signals that induce weak magnetic fields. MEG is a highly sensitive technology offering real-time assessment of neural activity as it measures these fields from outside the skull with a time resolution of milliseconds and a localization precision of some millimeters”, Fulford explains.
Nowadays, specialists from close to one hundred sites around the world have used this MEG technology to study and conduct research of conditions in patients diagnosed with neurological disorders. The TRIUX™ neo provides access to patients for the most precise information currently available on the market for functional brain mapping. It is used by neurosurgeons to support, e.g., surgical procedures for epilepsy and brain tumors in children as well as adults; applications of MEG are localizing important regions before surgical operations, localizing sources of epilepsy, and determining the function of various parts of the brain. Moreover, it is completely silent and non-invasive, making the experience very well tolerated by children. In addition to their HQ in Helsinki, Finland, MEGIN has local offices in the UK, US, and Japan.
A trusted partnership is been built by a long-term collaboration
Innokas Medical’s partnership with MEGIIN a neuroscience technology company in Helsinki, Finland has been based on a long-term collaboration which stems from previous corporation ownerships since 2007. Nowadays MEGIN is the global leader in magnetoencephalography (MEG) technology and launched in 2018 the fourth-generation MEG system, TRIUX™ neo.
“We’re holding the contract manufacturing responsibility of the system in our Helsinki factory, which is tailored for MEG device manufacturing – from electronics and control system manufacturing to final assembly, factory testing of both superconductive sensor elements and the whole MEG system and finally preparing the delivery to end-customers”, tells Ari Paalijärvi, COO at Innokas Medical.
“We see the cooperation with MEGIN significant for Innokas Medical as it supports our goal of continued growth also in the future”, he continues.
The manufacturing process of the device is accurate and complex, as the device technology is based on measuring the magnetic field of the brain with low-temperature superconductors. The measurement is done using over a hundred three-channel sensor elements contained in liquid helium. MEGIN’s Managing Director John Fulford says that they’re very happy with Innokas Medical’s industrial experience and expertise in manufacturing complex systems like TRIUX™ neo.
"The MEG system manufacturing requires knowledge of several special areas, such as cryogenics, micromechanics, computer techniques, and understanding of electromagnetic fields. As an organization, we’re satisfied with Innokas Medical's ability to support our production transfers of the products, build a state-of-the-art product, and equally provide flexibility for us as a business and our customers”, Fulford ponders.
“MEGIN's long-term collaboration with Innokas Medical has established a trusted partnership for both parties”, he concludes.
The availability of TRIUX™ neo is dependent on regulatory approvals. Please contact your MEGIN representative for details.
For more information about MEGIN, please go to:
For more information about TRIUX™ neo, please go to:
https://megin.fi/applications/