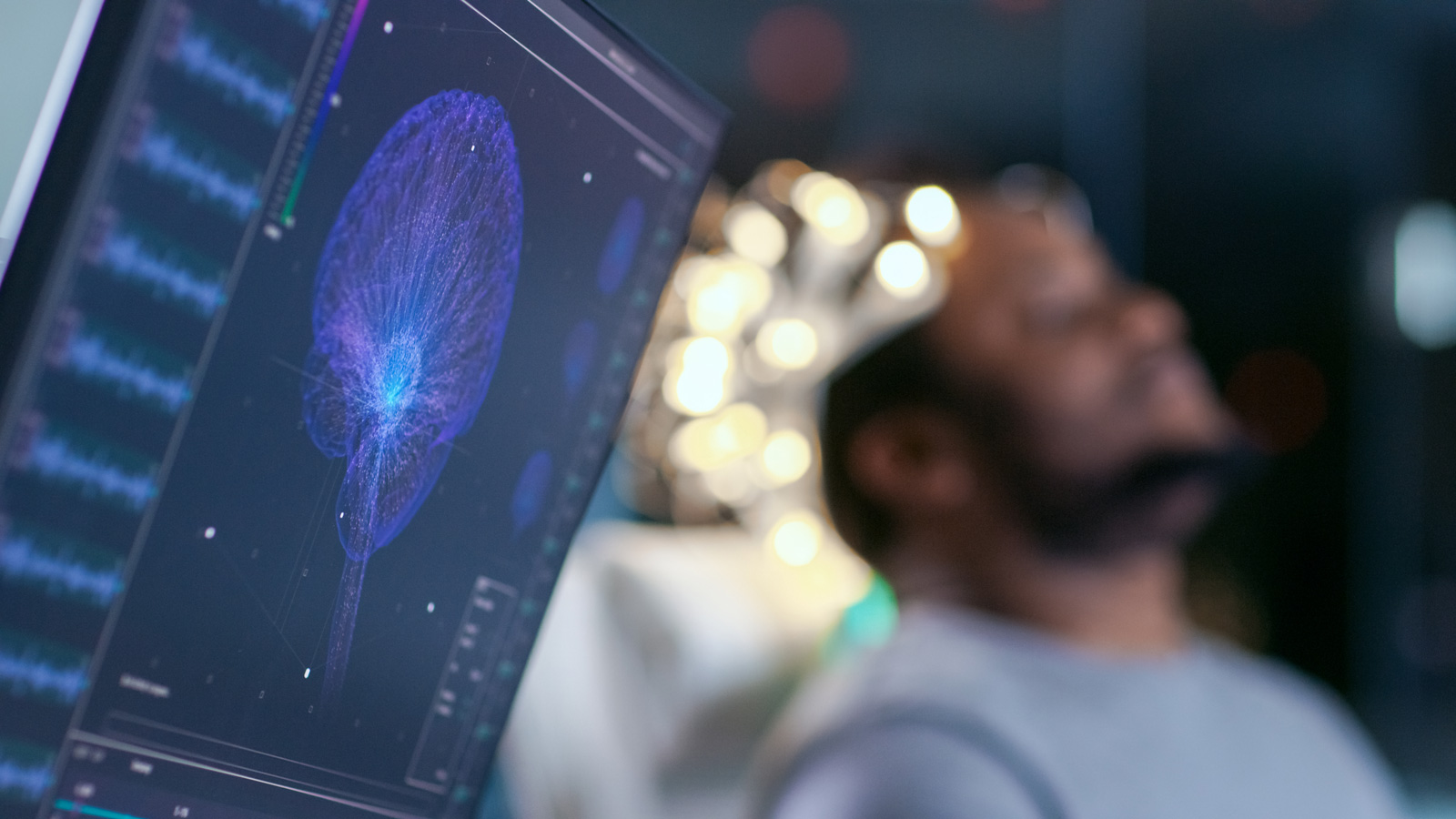
Terveystalo is the first Finnish healthcare service company to be granted the ISO 13485 quality management system certificate. The company invests heavily in the product development of intelligent...
Recent Posts
How do you solve the key challenges in turning a demanding technical solution idea into a tangible product and bringing it to the market? Could a capable contract designer and manufacturer help you...
Innokas Medical has joined the Finnish HL7 organisation in February 2022. Thus Innokas and especially its software house Digious continue to deepen their cooperation with the key industry players...
The 2022 Health Valley event is starting on March 15th and is accessible both live and online. The three-day healthcare innovation conference is taking place in Nijmegen, the Netherlands, collecting...
The Innovation Voucher, offered by Business Finland, is again open for application for Finnish SMEs, which seek to purchase new knowledge and skills for their business. The voucher supports companies...
The year is almost wrapped up and it’s time to take a breather and spend some time with our loved ones during the Holidays. The entire Innokas team would like to thank you for your cooperation and...
104 years - December 6th is a special day in Finland as we are celebrating Finland's Independence!
Hey there, we are going to attend Sweden’s largest happening for the manufacturing industry! Elmia Subcontractor is taking place as a physical and virtual event from November 9th to 12th! And the...
Last summer, Lybe Scientific got its first product registered as a CE-marked IVD medical device fulfilling the requirements set by In Vitro Diagnostic Directive (IVDD). The company built an ISO 13485...