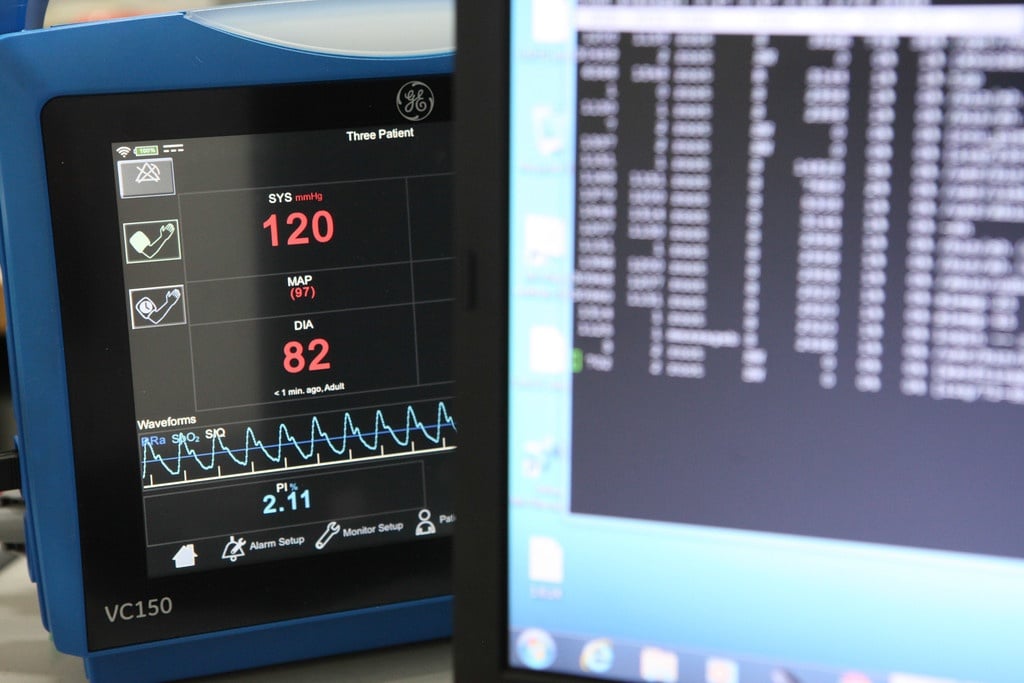
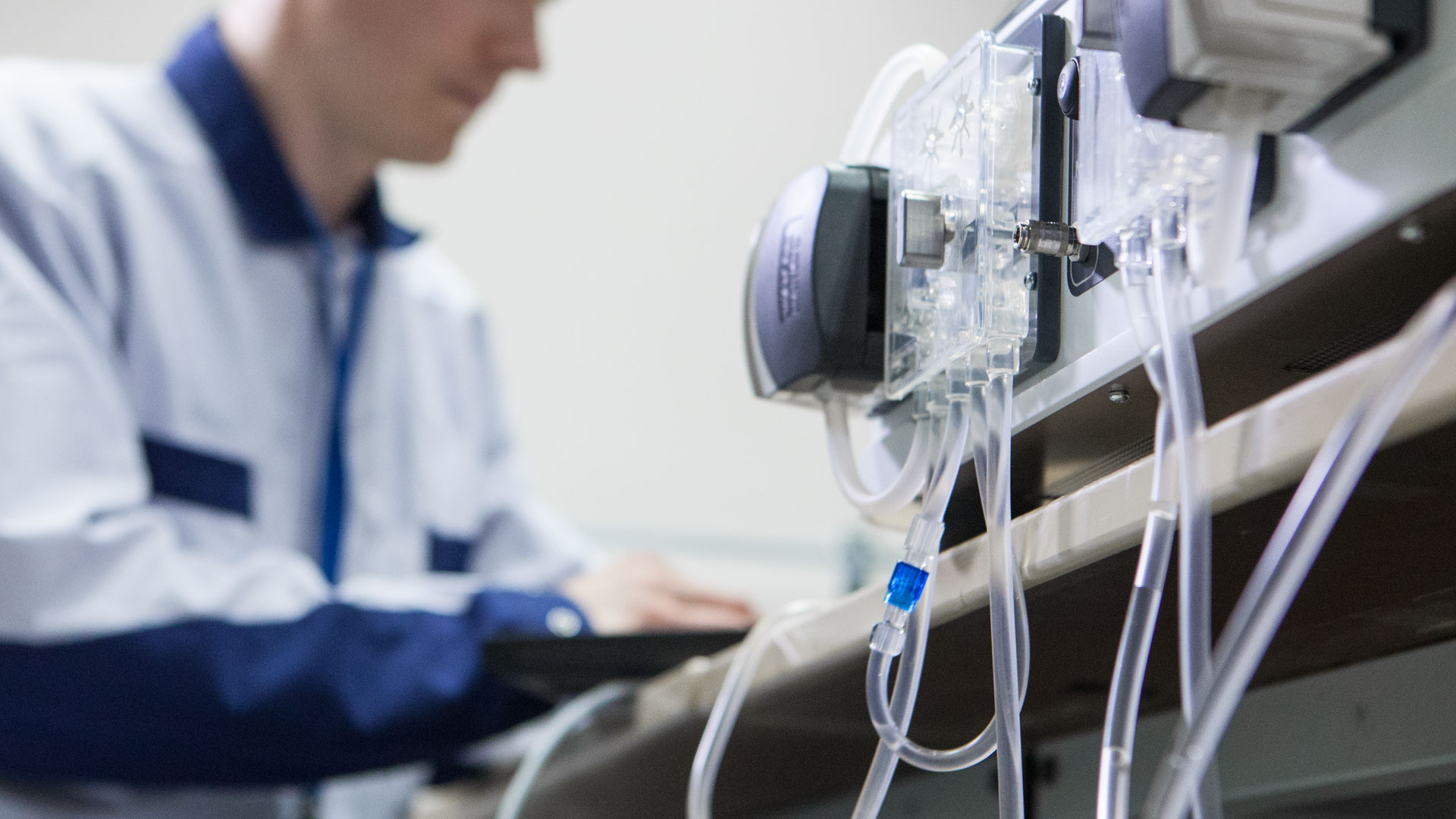
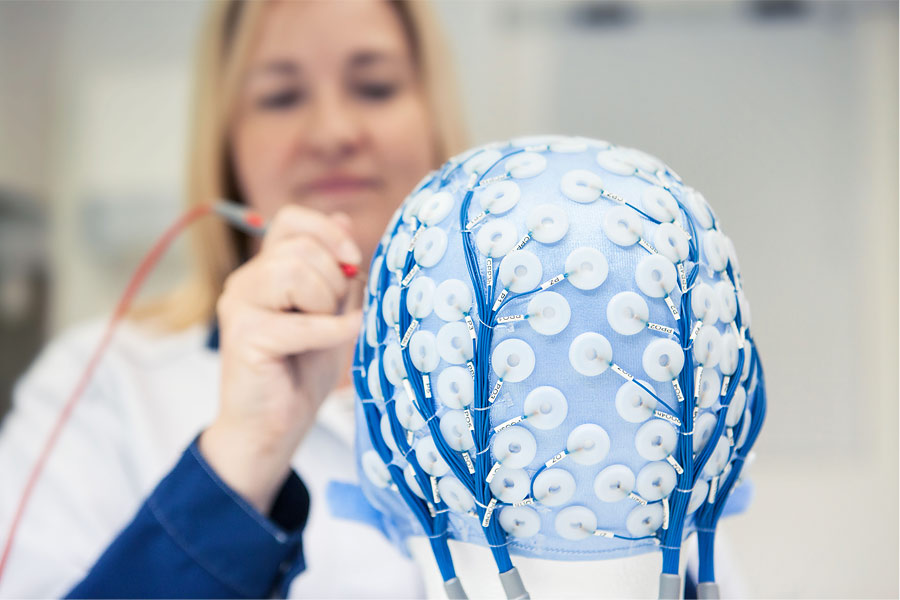
The software is becoming more and more important part of medical devices in the future. That’s why it is very crucial for the companies working in the field to be able to develop even more complex...
Recent Posts
Innokas Medical entered into cooperation agreement with Finnish company GrainSense Oy in 2016. The mutual interest for the companies is to develop a long-term, fruitful co-creation partnership, where...
Innokas will participate in two international trade fairs during the coming autumn: in Nordic Life Science Days (NLSD) event held in Stockholm, Sweden in the beginning of September, and in WHINN...
Across industries, the pace to introduce new products is increasing. This puts pressure to shorten the design and development cycles. Medical devices are no exception in this respect.
Healthcare technology is one of the largest high-tech export segments of Finnish industry, and the industry continues to grow strongly. According to the latest export report published by Healthtech...
Do you have an idea of a medical device? Or have you already started to develop your medical device? Do you want to enter, e.g., EU or US markets?
Finding answers? Welcome! - This is an unofficial guidance which is intended to assist medical device OEMs by answering to some common and basic questions concerning quality compliance and regulatory...
There’s few common trends in the medical field, which have a clear effect to the development of new medical products. The first one is the constantly changing jungle of rules and regulations, which...
Innokas Medical will participate in Taipuva ALM Solution Day which will be held in Helsinki, in Technopolis Aviapolis building, on 26th April. The topic of the day is to discuss about faster...