Across industries, the pace to introduce new products is increasing. This puts pressure to shorten the design and development cycles. Medical devices are no exception in this respect.
But one very common question in the medical field is that because of the burdensome regulations and more complex business environment, is it really possible to get certified, high-quality medical products to the market quickly and smoothly?
Yes, it is. We've found that agile medical device design and development is achievable despite the above-mentioned facts. One answer in shortening the development cycle as a whole is to apply three commonly known methodologies of product development to medical device development: Design Thinking, Lean Start-up, and Agile.
But how these methodologies can be applied to medical device development, and how this shortens the product development cycle? You really should continue reading now, as the answers are right behind the corner!
Short introduction to the three methodologies
Let’s first discuss a little about these three methodologies. Design Thinking, Lean Start-Up and Agile are common approaches to develop digital services. There are numerous representations on how these approaches co-exist and how they help in reducing the development times by eliminating waste and focusing on the essentials any given time. You can see one example of the presentation when developing digital services below (picture #1).
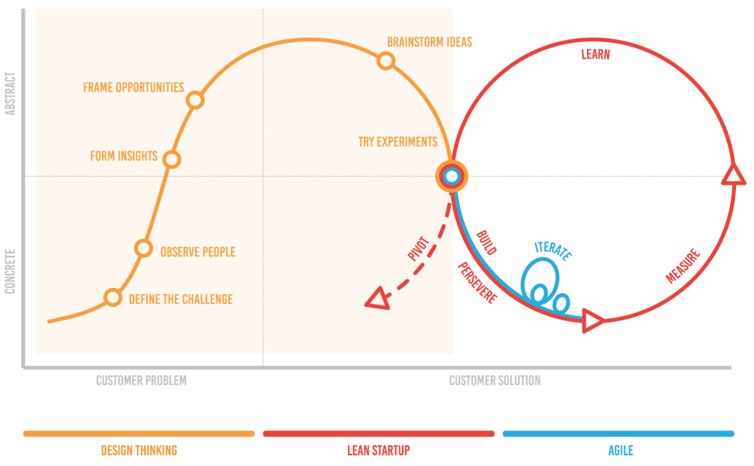
Picture #1. Example of Design Thinking, Lean Start-Up and Agile approaches - how they co-exist and how they help in reducing the development times by eliminating waste and focusing on the essentials any given time. Source: Servian / Nordstrom Lean Model.
Design Thinking utilizes elements from the designer's toolkit like empathy and experimentation to arrive at innovative solutions. It strongly emphasizes the user or human-centric perspective in the design process. Design tools and approaches are systematically used to come up with innovative design solutions satisfying the user expectations.
Lean Start-Up methodology aims to shorten product development cycles by adopting a combination of business-hypothesis-driven experimentation and prototyping, iterative product releases, and validated learning. The central hypothesis of the lean startup methodology is that if companies invest their time into iteratively building products or services to meet the needs of early customers, they can reduce the market risks and sidestep the need for large amounts of initial project funding and expensive product launches and failures.
Agile is an umbrella term borrowed from SW development but having a wider applicability in which cross-functional teams work with users to develop and release MVP’s frequently in short cycles. Agile encourages collaboration and self-organization among multidisciplinary team members.
Design Thinking, Lean Start-Up and Agile methodologies in medical device design and development
According to our studies, medical device development can greatly benefit from these three, commonly known methodologies of product or service development. The following figure describes how the three approaches interact and how they can be applied in medical device design and development.
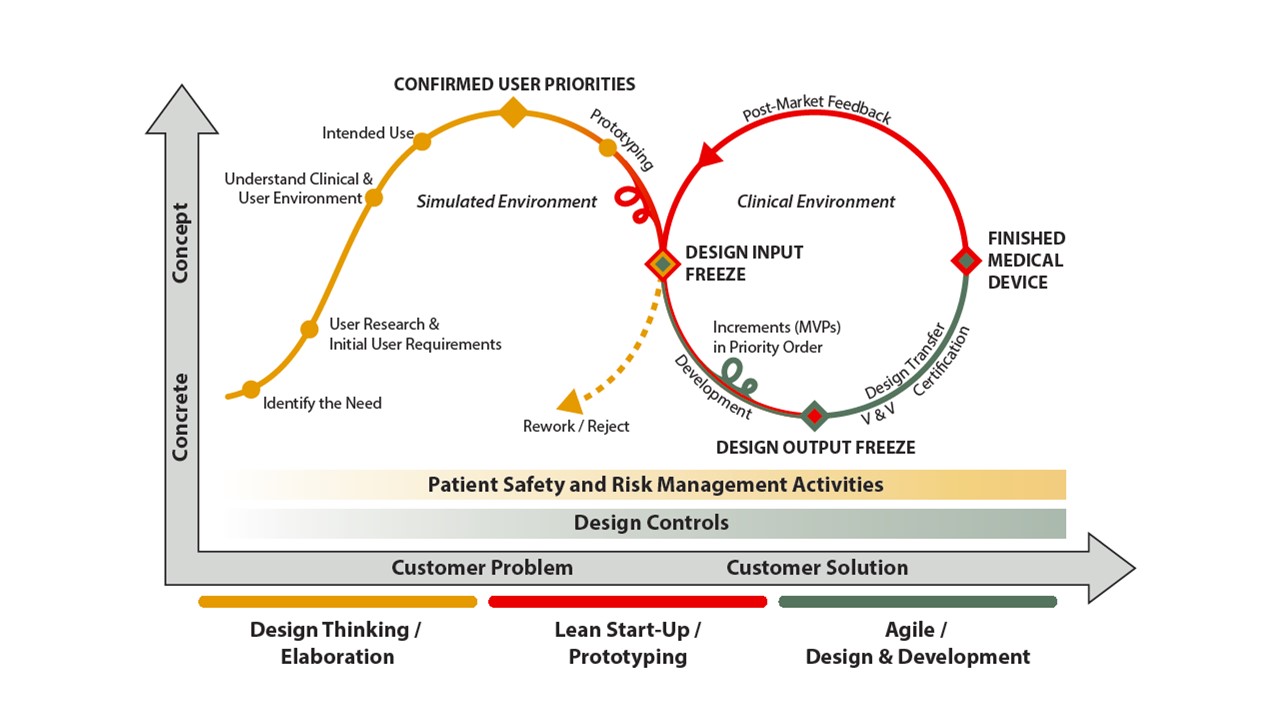
Picture 2. Design Thinking, Lean Start-Up and Agile methods in Medical Device Design and Development.
Design Thinking is something that can be utilized especially during the very early phase of the medical device development – at the idea elaboration phase. It is a powerful approach when defining the design challenge and seeking the answers to the questions: “What problem are we trying to solve?” and “What are the real user needs?” Besides the initial user requirements, understanding the clinical and user environments as well as the intended use are also crucial when developing medical devices.
Lean Start-Up methodology is something that can be utilized especially during the prototyping phase, aiming to shorten product development cycle. Fast prototyping as well as combining insights and skills from various essential domains in medical device development brings additional understanding about the feasibility of different alternatives.
Agile is something that can be utilized especially during the actual design and development phase. Agile is a good approach for incremental development and testing, which both are done in short iterations. The four values of agile (see e.g. Agile Manifesto), are relevant for the medical devices as well. The values are represented and discussed more in our White Paper, which can be found also from the end of this post.
The benefits of applying the three methodologies in medical devices design and development
To achieve more agile medical device design and development cycle, co-creation and standardized working methods are something that should be incorporated. In addition, the above discussed three methodologies are something that can be applied to the development process to achieve that. When done properly, applying these three methodologies to medical device design and development will reduce the time to market of the device by ensuring that right and well specified customer problem will be solved with a feasible solution, which is going to be incrementally developed.
However, there are some notable differences that must be accounted for so that these approaches will work seamlessly together and add business value. E.g., aspects that are typical to the medical devices domain shall be taken into account when designing the actual life-cycle model. The most important being the Design Controls as well as patient and user safety via Risk Management.
The discussion above related to the three methodologies in medical device design and development was only a small view to the topic. If you want to learn and read more, we've now created a Free White Paper, in which we’ll describe in a very detailed level how to get high-quality medical products to the market as quickly as possible by utilizing product development model, which is based on these three methodologies. The illustration above (picture #2) will be also described and discussed from checkpoint to checkpoint. Collect yours by following the link!











