According to the latest export report published by Healthtech Finland, the exports of the Finnish health technology reached again a new record in 2020.
Recent Posts
Knowledge-based management is on today’s agenda in many organizations; how to derive insights from the large datasets to enable better and faster decision-making. The role of digital health...
Mr. Janne Kostamo (Executive MBA, M.Sc. in Industrial Eng. and B.Sc. in Mech. Eng.) was appointed as new CEO of Innokas Medical. He started to work at Innokas in the beginning of this year. Kostamo...
Enabling scalable and cost-efficient future healthcare solutions with autonomous health monitoring is the most important driver for Medicubex, headquartered in Helsinki, Finland. Innokas Medical is...
Three manufacturing sites offer flexible contract manufacturing and agile capacity increasing adjusting to customer-specific needs.
FlowOx 2.0, developed in co-creation between Norwegian company Otivio AS and Innokas Medical, is a clinically promising, patented and cost-efficient home treatment solution for Peripheral Arterial...
The Innovation Voucher, offered by Business Finland, is one funding opportunity for Finnish SMEs who seek for purchasing new knowledge and skills for their business. According to Business Finland,...
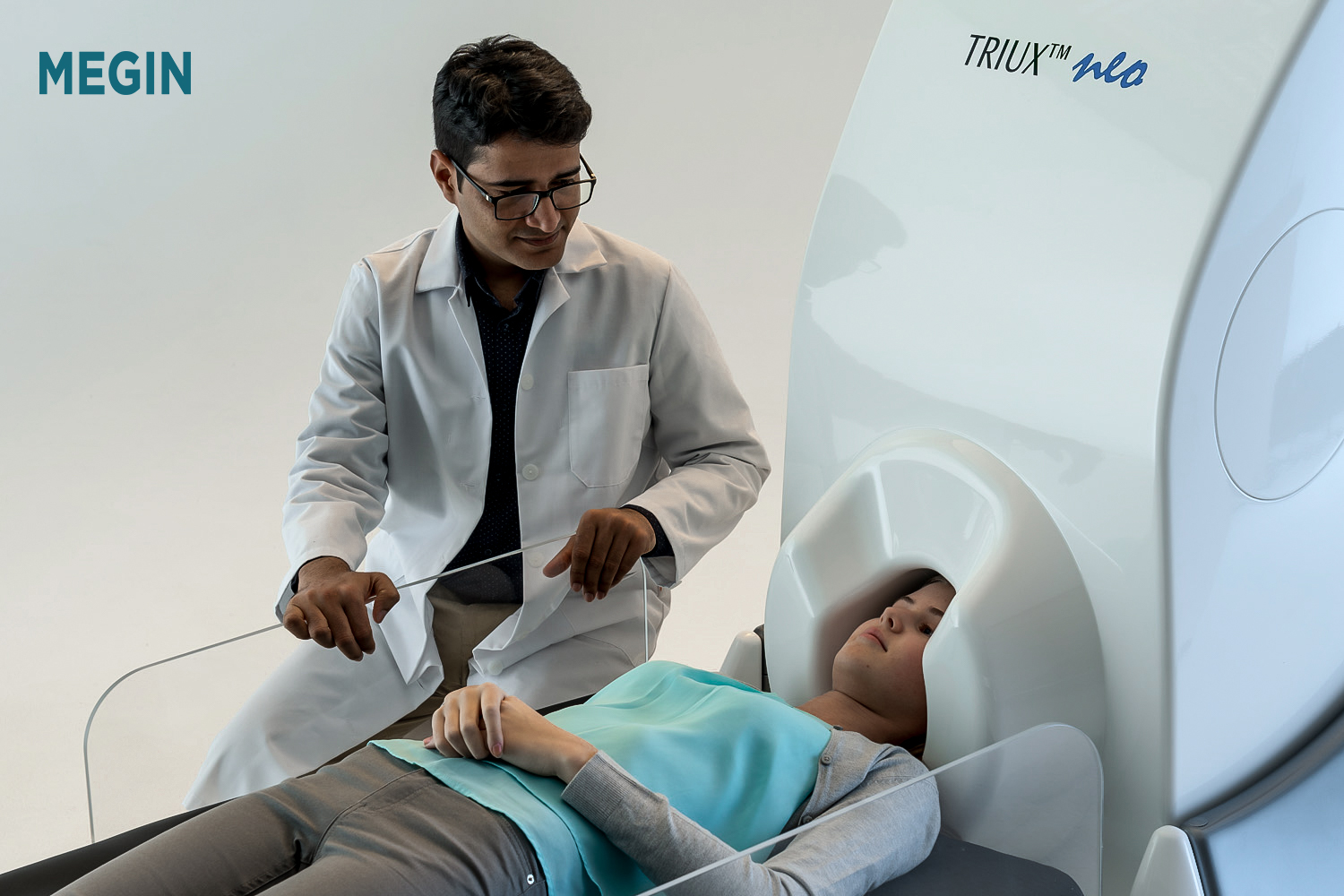
Headquartered in Helsinki, Finland, MEGIN is the global leader for magnetoencephalography (MEG) technology used for their non-invasive functional brain mapping solution. A long-term collaboration...
Innokas Medical, with expertise dedicated to medical devices and software, and Biocodex PhaMe, providing pharmaceutical consultant services, have agreed on a strategic partnership. Through this...