Last year Innokas launched a new strategy to seek strong growth. This means we are extending our presence into new market areas as well as developing our services and launching new ones. In 2022 we...
Recent Posts
Terveystalo is the first Finnish healthcare service company to be granted the ISO 13485 quality management system certificate. The company invests heavily in the product development of intelligent...
Innokas Medical has joined the Finnish HL7 organisation in February 2022. Thus Innokas and especially its software house Digious continue to deepen their cooperation with the key industry players...
Healthcare technology is one of the largest high-tech export segments of Finnish industry, and it continues to grow strongly year after year. In addition to growth, the industry is evolving with...
Healthcare technology is one of the largest high-tech export segments of Finnish industry, and it continues to grow strongly year after year. In addition to growth, the industry is evolving with...
The collaborative consortium has received a significant funding from Business Finland. This two-year Stroke-DATA -project, which is one part of Business Finland’s Smart Life program, is carried out...
The importance of SW is growing – also in medical devices and as medical devices. This blog text deals with the change of SW’s role, the tightening regulations landscape as well as how to be agile...
Innokas Design Studio develops software for regulated medical devices to improve peoples' well-being
Healthcare technology is one of the largest high-tech export segments of Finnish industry, and the industry continues to grow strongly year after year. In addition to growth, development is also...
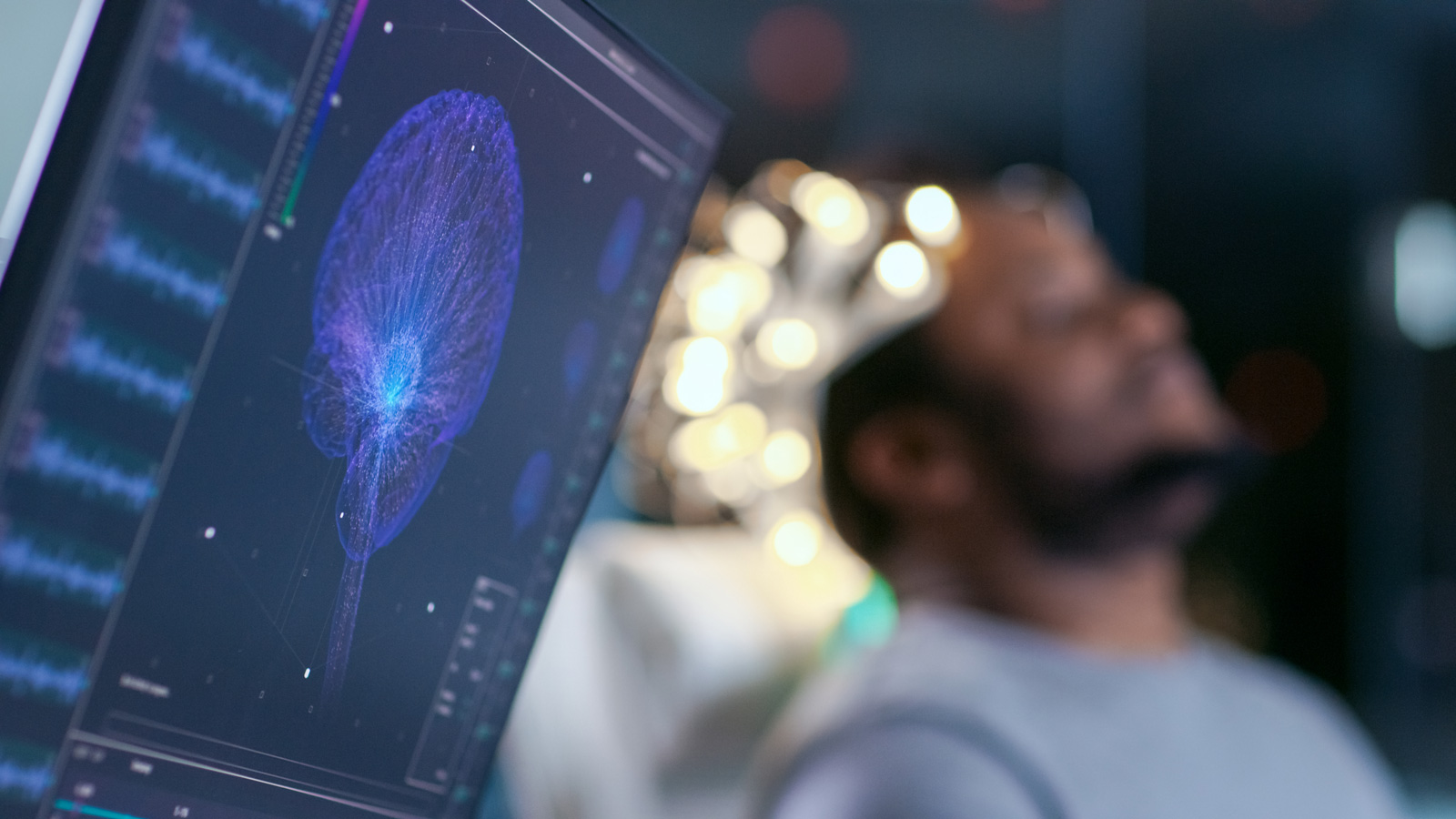
We’re living in an amazing world where the new technologies and the science itself enable new kinds of miracles happen in saving peoples’ lives and in improving their well-being. In recent years, one...