Innokas was chosen by Orla DTx to design and develop a software library for asthma-related calculations to ensure their product meets MDR requirements as a Class IIa medical device. The collaboration...
Recent Posts
Complex product development projects are dynamic and require adaptability to maintain control over timelines and budgets while building the winning product. Teaming up with an experienced partner...
Innokas, a responsive and adaptive technology partner, collaborated with Sartorius, a global leader in Liquid Handling and In-Vitro Diagnostic (IVD) devices, on a significant project. This endeavor...
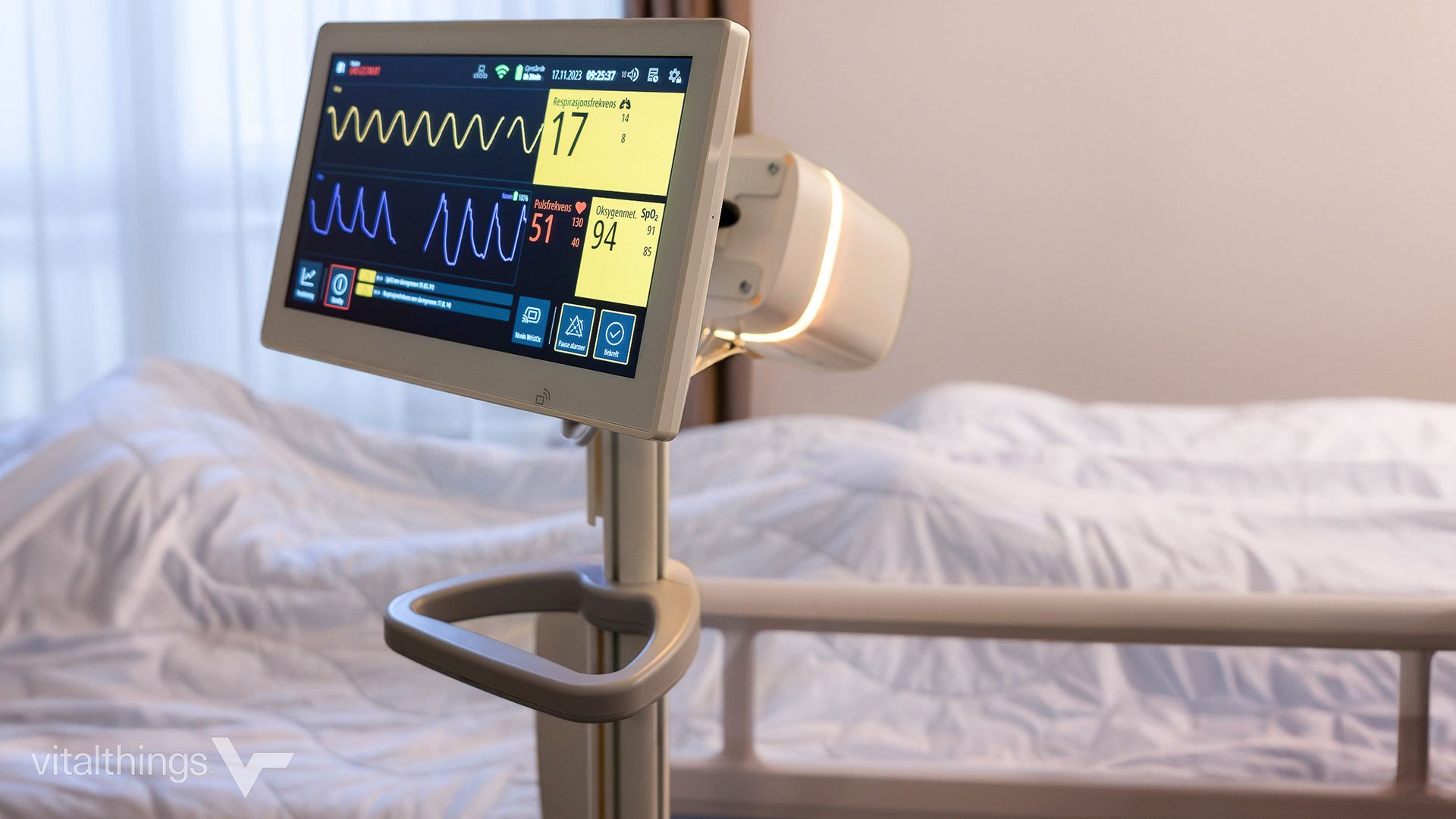
Pictured: Innokas product developer Antti Tuovinen and marketing specialist Teija Tulinen with the patient monitor. Antti Tuovinen works as an electronics engineer in the Glucoset project
Creating...
Combining different devices together into smooth, uniform, and easy to use combination takes special kind of expertise. That kind of expertise was showcased in Teknologia 23 event in Helsinki Expo...
Outwardly, the device looks like a conventional office soundproof telephone booth. In reality, it’s an autonomous health self-measurement station, empowering users to independently assess their...
Terveystalo is the first Finnish healthcare service company to be granted the ISO 13485 quality management system certificate. The company invests heavily in the product development of intelligent...
Last summer, Lybe Scientific got its first product registered as a CE-marked IVD medical device fulfilling the requirements set by In Vitro Diagnostic Directive (IVDD). The company built an ISO 13485...
Innokas Medical entered into cooperation agreement with Swedish company C-RAD AB in 2016. The mutual interest for the companies has been to develop a long-lasting partnership built on trust and with...