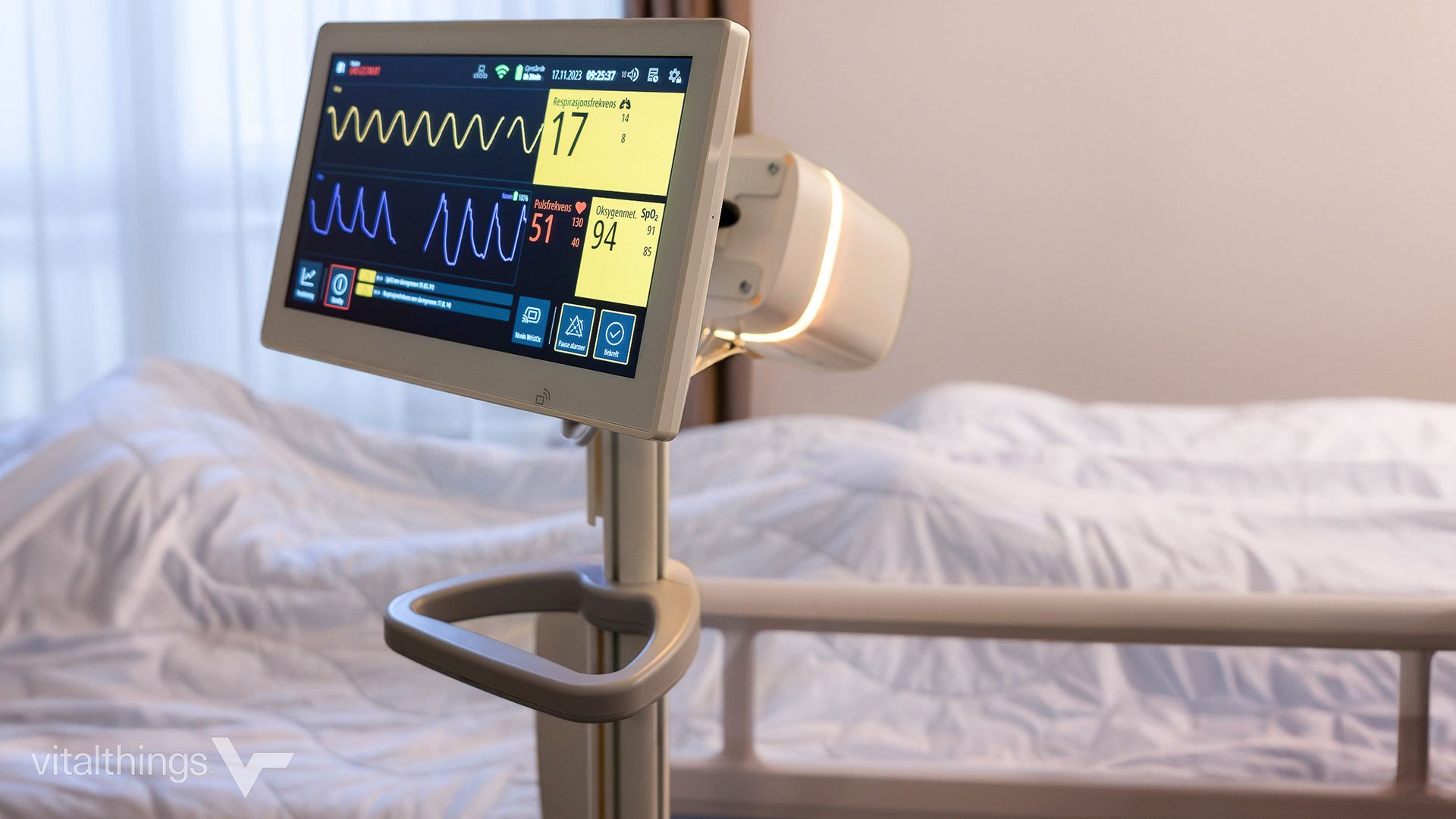
Developing medical or other complex devices starts with getting the design right. Early design decisions lays the groundwork for efficient manufacturing and faster time to market. One of the biggest...
Recent Posts
Moving production from one factory to another is a complex process that requires careful planning and execution. A new, competent manufacturing partner should ensure that the transfer goes as...
Greetings from the “Husky Company” you might have heard of at DMEA 2025. Our eventgoers Visa Poikela and Päivi Leppänen discussed the event's quality and networking opportunities and highlighted the...
Innokas was chosen by Orla DTx to design and develop a software library for asthma-related calculations to ensure their product meets MDR requirements as a Class IIa medical device. The collaboration...
Complex product development projects are dynamic and require adaptability to maintain control over timelines and budgets while building the winning product. Teaming up with an experienced partner...
Sustainable product design is increasingly expected aspect in product development. Stricter environmental regulations, consumer demand for eco-friendly products, and investor support for sustainably...
While artificial intelligence is a highly anticipated tool for healthcare, its widespread adoption calls for experimentation, responsible development, and strict regulation to an extent that’s less...
It’s common practice in many industries to have prototypes made in different location than where mass production is planned. However, this approach can slow down production ramp-up and negatively...
Tomi Hugg has recently been appointed as the new Director of Design Services at Innokas. "The deep technological expertise at Innokas across industries was a major reason for my decision to apply,"...