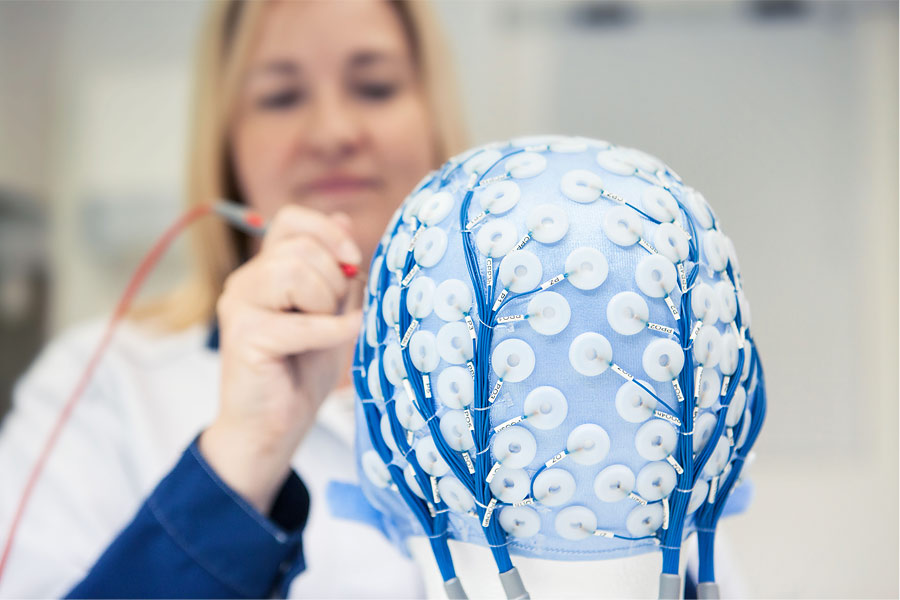
There’s a few common trends in the medical field, which have a clear effect to the development of new medical products. The first one is the constantly changing jungle of rules and regulations, which...
Recent Posts
MedTech as business is changing and getting more complex. For example, the technical complexity and regulatory requirements are something that are increasing as we speak. In addition, the user...
MedTech as a business is changing and getting more diverse. For example, the technical complexity, forms of digitization and regulatory requirements are something that are increasing as we speak. At...
MedTech as business is changing and getting more complex. For example, the technical complexity and regulatory requirements are something that are increasing as we speak. At the same time there is a...
MedTech business is changing and getting more diverse. The technical complexity, caused mainly by digitization, is increasing. New technologies are being introduced at faster pace, the impact of...
MedTech as a business is changing and getting more diverse. For example, the technical complexity, forms of digitalization and regulatory requirements are something that are increasing as we speak....
Before introducing new medical devices to the market, there’s much more than simply coming up with an innovative idea, building it, and offering it to the masses. One clear rule in the medical field...
MedTech as a business is changing and getting more complex. For example, the technical complexity and regulatory requirements are something that are increasing as we speak. At the same time there is...
There’s few common trends in the medical field, which have a clear effect to the development of new medical products. The first one is the constantly changing jungle of rules and regulations, which...