MedTech business is changing and getting more diverse. The technical complexity, different forms of digitalization and related regulatory requirements are increasing as we speak. And there is the eternal pressure to lower the costs, improve quality and get to market with new innovations even faster. We argue that every medical device OEM who is designing and developing medical devices has faced or will face these issues.
Thus, we at Innokas are developing the best practices, which ensure smoother, more agile and shorter development cycles for certified, high quality medical devices. And now we want to share our advices to help you to choose the best possible partner to get your medical innovations to the regulated markets as quickly as possible.
Our advices are:
Advice #1. Ensure the standard compliance already from the start of the development
Advice #2. Change your mindset to enable more agile path
Advice #3. Joint processes uniting wide expertise are truly needed
Advice #4. Many kinds of knowhow and competences are required - ensure the expertise
Advice #5. Modern tools can make a difference
Advice #6. Co-creation is not enough. You’ll need some co-creation models, too.
Advice #1. Ensure the standard compliance already from the start of the development
We’d like to emphasize that before introducing new medical devices to the market, there’s much more than simply coming up with an innovative idea, developing it, and offering it to the masses. It must be recognized early on that the medical device design and development process must meet very strict criteria stipulated in the ISO 13485 Quality Management System (QMS). It must meet the requirements set by the regulatory authorities of intended market areas, e.g. FDA for United States. There are important areas governed by specific standards, as well – like process related standards (e.g. ISO 14971 for Risk management, IEC 62366 for Usability engineering and IEC 62304 for Software life cycle processes) and device related standards (e.g. IEC 60601 for the safety and essential performance of medical electrical equipment). The design of the device must satisfy all the criteria if the development is to proceed.
The rules and regulations kick in as soon as you come up with the new idea; the regulations for the whole product life cycle must be recognized already in the early ideation phase. This is because, e.g., the intended use and the classification of the device have a significant impact on the laws, standards and regulations as well as on the documentation that the authorities require. Our recommendation is to put strong focus on the idea and conceptualization phase, because with the right choices made already at that phase, the more agile project schedules can be achieved later in the development.
All this puts medical device development teams in a very demanding position. There is A LOT to know and to be taken care of in the design and development phase of the product. And if the company lacks the knowledge, the development process itself will be long and rocky and in the worst-case scenario, the product will not be eligible to enter the target markets at all. One way to deal with this is to partner with some professional that can help you to proceed with all the Quality and Regulatory actions. Alternatively, you can outsource the design, development and/or manufacturing to a partner company who has the needed experience and knowledge already in-house and the effective QMS in place.
Advice #2. Change your mindset to enable more agile path
Taking the rules and regulations into account already in the idea phase - and throughout the device life cycle - isn’t, of course, enough. There’s much more to get your high quality, regulated medical device to the market as quickly as possible.
Our next advice is to change your mindset to co-creation and building it on the best ways of working. This is because by co-creating, companies can take the challenges of more complex operative environment – including quality and regulatory actions - into account more effectively and efficiently. Co-creation is the future way of creating MedTech products and services.
But how the co-creation mindset and way of working in the MedTech world should look like? We have a vision on that. We’ve started a discussion where we state that one answer to shortening the development cycle of medical products is to apply three commonly known methodologies of product development to medical devices: Design Thinking, Lean Start-up, and Agile. According to our studies, medical domain can greatly benefit from these three methodologies by incorporating the customer’s voice as well as speed and agility from the development of digital services, where these methodologies have been widely used over the past years. Our vision emphasizes co-creation throughout the whole design and development phase.
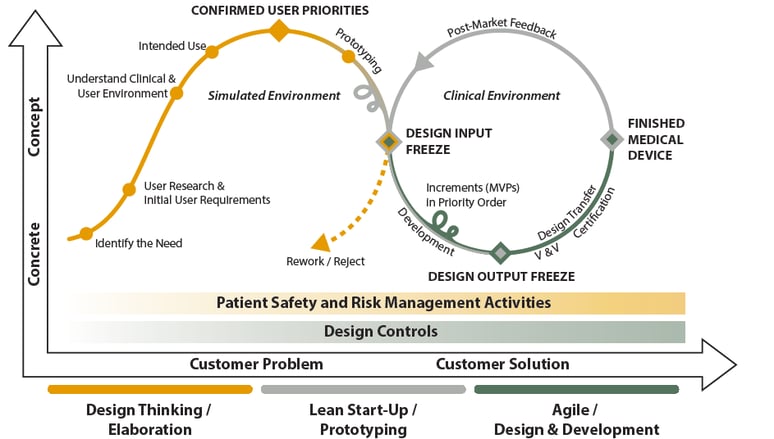 To learn more about the application of these methodologies in medical environment, please collect your White Paper from the end of this blog post!
To learn more about the application of these methodologies in medical environment, please collect your White Paper from the end of this blog post!
We see that this kind of renewed medical device development model results in more value-add for the development investment. When done properly, it reduces the time to market by ensuring that the right and well specified customer problem will be mutually solved with a feasible solution, which is going to be incrementally developed.
Advice #3. Joint processes uniting wide expertise are truly needed
Joint processes are another important element of the effective product development. They ensure involvement and co-operation of a wide team of experts in the project throughout the design and development.
They unite the experts from design and development, quality and regulatory as well as manufacturing teams. When thought through and executed properly, these cross-functional teams ensure holistic view and more agile progress from idea through development to design transfer and manufacturing. It also guarantees that all the quality and regulatory requirements as well as manufacturing needs are considered already in the early phase – which naturally shortens the development cycle of the product.
The joint processes must cover all the areas of medical device design and development. They also should provide common concepts and terminology for all the parties. In this way, the development process is more involving. By designing the stages and stage gates carefully, freedom to brainstorm and experiment can be encouraged in the early phases, and more design controls can be added towards the end of the process based on solid reasoning.
Our suggestion for you is to check out that your partner has the joint processes uniting wide team of experts in place and that they incorporate the necessary design controls applied at the right process steps. This will make a huge difference.
Advice #4. Many kinds of knowhow and competences are required - ensure the expertise
It’s clear that many kinds of knowhow and competences are required from medical device development teams. That’s why we see that one key element in agile co-creation is an extensive medical device design and development expertise in-house, or at your co-creation partner, to generate common knowledge and assets for the whole team.
There are many ways to solve this, but as an example, at Innokas Medical this is solved by establishing the Network of Chiefs. Chief role is something that has been established for relevant subject matter areas to support effective ways of working.

The Chiefs, e.g., actively follow and contribute to the development of their own domain, support the development projects and share information with colleagues in their own domain. They’re also creating and providing insights and making them easily available for all – including, and especially, our customers.
We see that the network of Chiefs is very important when aiming to establish new ways to co-create with the customers and shorten development cycles in practice. Thus, we suggest you evaluate the competences and expertise your partner really has in-house.
Advice #5. Modern tools can make a difference
To enable systematic and efficient co-creation and reuse of accumulated knowledge, modern tools - which are also aligned with the joint processes and with the vision - are truly needed. One good example of this is ALM (Application Lifecycle Management) tool, which enables – among other things – traceability, design controls automation, status reporting as well as accumulation of information and its reuse.
For example, at Innokas our Customer Project Template, that is configured in our ALM tool, provides the common approach and platform for all customer projects. It covers around 95 % of the project needs and it is easy to create project specific configuration to cover the rest. It also automates the fulfillment of design controls and regulations. Customers can be granted access to the tool allowing a seamless co-creation e.g. when refining and reviewing requirements.
Thus, when selecting the partner, ask them to show and explain you the tools they’re using. Tools can really make the difference. But also remember, that it is not just the tools that are important, it is HOW they are used, trained and kept up-to-date with changing business and regulatory requirements.
Advice #6. Co-creation is not enough. You’ll need some co-creation models, too.
It is not enough to work closely together. We see that it is important to set up the best customer co-creation models, which will enable the early and close involvement of the customer, the transparency throughout the whole development process and the real-time communication between parties. This will enable the co-operation parties have mutual understanding of the problem to be solved, which sets the direction for next steps crystal clear and caters the way to business success.
Additionally, the project steering, requirements elaboration and approvals can be conducted in more agile and co-operative ways when using modern tools together. We at Innokas are also convinced that using focused workshops, partner networks utilization and QA&RA support in the projects have significant positive impact on project schedules.
Take your time to evaluate, which kind of co-operation models your partner uses.
Conclusion: co-creation brings advantages for everyone
As stated above, we see that the best business results will be achieved through the value add of strategic co-creation partnerships, where the modern working methods and tools, wide range of expertise, fine-tuned joint processes as well as customer co-creation models are brought together. Through this kind of co-creation partnership, every party can ensure that all the business-related complexities are solved, while the regulatory requirements are met during each life cycle phase of the product - at the same time speeding-up the market launch and reducing the total costs of the product.
Here are our “two cents” for choosing your partner wisely. Ask your partner candidates to show you the concrete benefits they can deliver to your business. This is the way to save both money and time when developing next generation medical devices.
PSST! The discussion above related to the three methodologies in medical device design and development was only a glimpse to the topic. If you want to learn and read more, we've created a Free White Paper, in which we describe in more detail how to get high-quality medical products to the market as quickly as possible by utilizing product development model, which is based on Design Thinking, Lean Start-Up and Agile. The illustration above (picture #1) will be also described and discussed from checkpoint to checkpoint. Collect yours by following the link!











