Across industries, the pace to introduce new products is increasing. This puts pressure to shortening the design and development cycles. Medical devices are no exception in this respect.
Design Thinking, Lean Start-Up and Agile are common approaches to develop digital services. There are numerous representations on how these approaches co-exist and how they help in reducing the development times by eliminating waste and focusing on the essentials any given time. This is something that companies in the medical field seek as well; how to get certified, high quality medical product to the market as quickly as possible?
We’ve found that agility can be achieved in medical business despite the burdensome regulations and more complex business environment. Co-creation mindset is one answer to that – in addition, we’ve applied these commonly known three methodologies – Design Thinking, Lean Start-up and Agile – to medical environment. Medical domain can greatly benefit from these three methodologies.
Design Thinking, Lean Start-Up and Agile methods in Medical Device Design and Development
According to our studies, Design Thinking, Lean Start-Up and Agile methodologies can be successfully applied to the design and development of medical devices. When done properly, they reduce the time to market of the device by ensuring that right and well specified customer problem will be solved with a feasible solution which is going to be incrementally developed.
The following figure describes how the three approaches interact and how they can be applied in medical device design and development.
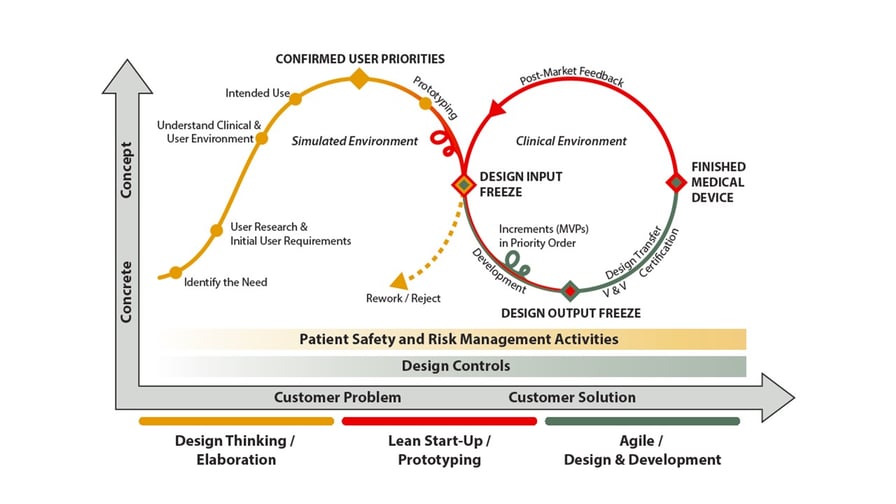
Design Thinking, Lean Start-Up and Agile methods in Medical Device Design and Development.
When comparing the figure to other industries, we can see that there are some notable differences that must be accounted for so that these approaches will work seamlessly together and add business value. The imperative need for quality management system, as well as patient safety and risk management must be taken seriously into account. These determine the design and development of medical devices right from the idea phase, with increasing intensity.
Effective medical device design and development requires deep understanding about the users, clinical environments and technologies to be used as well. The team needs to have a broad spectrum of competences where some of those are specific to the medical domain.
Learn more about Design Thinking, Lean Start-Up and Agile Approaches in Medical Device Design and Development
There is a pressure to get high-quality medical devices quickly to the market, and this pressure is common for everyone in the field. This is why we wanted to create a dedicated White Paper, which elaborates in detail how these three methods can benefit the design and development of medical devices.
The following topics are covered in the white paper:
- Mapping of medical device development concepts into the spectrum of the three mutually supporting approaches (Design Thinking, Lean Start-Up and Agile).
- Strong and imperative focus on patient safety and related product risk management
- The role of design controls throughout the development lifecycle
- The challenge related to the concept of Minimum Viable Product (MVP) in a regulated environment.
Please, download your free White Paper below!











