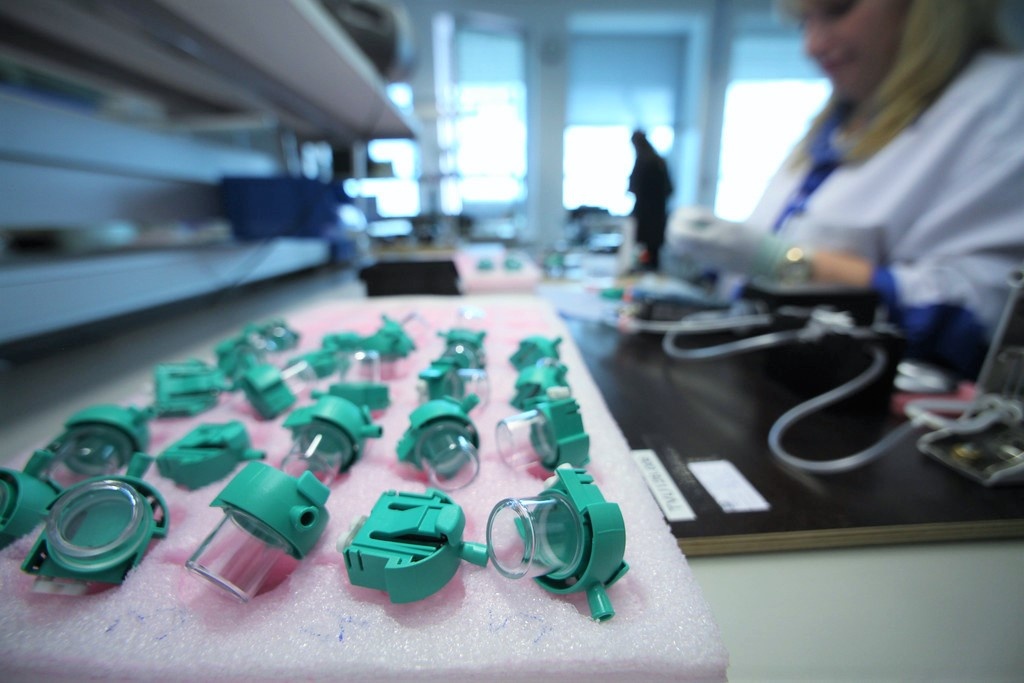
D-fend Pro Water Traps are designed to protect patient monitors from moisture, humidity and contaminants. Innokas Medical is GE Healthcare’s contract manufacturing partner, producing the product to GE at its Tallinn factory with high annual production volumes.
GE Healthcare provides transformational medical technologies and services to meet the demand for increased access, enhanced quality and more affordable healthcare around the world. GE (NYSE: GE) works on things that matter - great people and technologies taking on tough challenges. From medical imaging, software & IT, patient monitoring and diagnostics to drug discovery, biopharmaceutical manufacturing technologies and performance improvement solutions, GE Healthcare helps medical professionals deliver great healthcare to their patients.
Innokas Medical and GE Healthcare have cooperated for several years already. Innokas has worked together with GE Healthcare in product development and engineering as well as in manufacturing for example within patient monitoring, mammography and nuclear medicine. In addition, Innokas has offered its product life cycle management as well as quality and regulatory services to GE Healthcare.
One form of cooperation between Innokas and GE is located in Innokas Tallinn low-cost factory. Innokas and GE Healthcare have worked closely together with optimizing the effectiveness and cost-efficiency of the mass-production of GE’s D-Fend Pro Water Trap, which is CE-marked and cleared by the FDA. Water Traps are designed to protect the patient monitors from moisture, humidity and contaminants, which can harm the respiratory monitoring and affect measurement accuracy. The product is used with GE’s respiratory modules in anesthesia and critical care applications. Innokas Medical manufactures the product for GE Healthcare at the Tallinn factory with high annual production volumes.
“Although D-Fend Pro Water Trap is a fairly straightforward disposable accessory, its role in the success of the patients’ respiratory monitoring is significant. We’ve required high-quality and uniform products from Innokas, flexibility to meet the volume change requests and the ability to improve the production efficiency. We have been very happy with Innokas Medical’s way of operation as we can together improve the production process”, says Terho Hoskonen, Managing Director of GE Healthcare Finland Oy.
According to Jouni Toijala, CEO at Innokas Medical, one of the best effects of the manufacturing cooperation of the product has been the ability to develop Innokas’ skills through the joint development of operations. Innokas and GE Healthcare have for example substantially increased the annual production capacity of D-Fend Pro Water Trap by developing the manufacturing process of the product. As a key components in the development of the mass production and capabilities have been the development made with production lines, tools, testers and basic equipment at the Tallinn factory. Through these, a significant improvements in the mass-production effectiveness of the product have been achieved. In addition to this, through streamlining and developing the production operations, Innokas has been able to reduce the production costs of D-Fend Pro Water Trap continuously.
“Due to the long-term, deep co-creation partnership between GE Healthcare and Innokas Medical, we’ve been able to enhance and develop our capabilities within mass-production, production capacity increasing and cost-efficiency. It is a point of honor for us to act as a manufacturing partner for GE Healthcare and take care that the manufacturing processes related to their products follows all the required directives and regulations. The cooperation between GE Healthcare and Innokas Medical is still ongoing today, and the mutual benefit of the cooperation will surely grow also in the future”, Toijala tells.